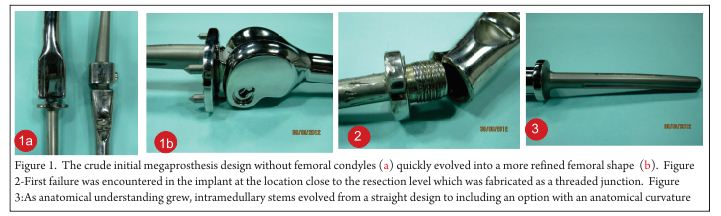
Current role of FDG-PET in Bone and Soft tissue tumors
Vol 1 | Issue 1 | May – August 2015 | page:29-36 | Junaid Ansari[1], Reinhold Munker[1], Amol Takalkar[2,3*].
Author: Junaid Ansari[1], Reinhold Munker[1], Amol Takalkar[2,3*].
[1]Feist Weiller Cancer Center, Shreveport, Louisiana.
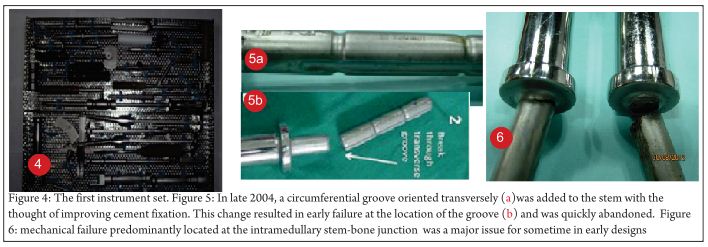
[2]Center for Molecular Imaging & Therapy, Biomedical Research Foundation of Northwest Louisiana.
[3]Dept. of Radiology, LSU Health, Shreveport, Louisiana.
Address of Correspondence
Dr. Amol Takalkar MD.
Dept. of Radiology, Louisiana State University Health Sciences Center – Shreveport, 1505 Kings Highway, Shreveport, LA 71103
Email: atakalka@biomed.org
Abstract
FDG-PET/CT imaging is an established modality for the workup of several malignancies; it is now considered standard for the initial as well as a subsequent treatment strategy in the management of most malignancies. The focus of this article is to discuss the role of FDG-PET/CT imaging in the workup and management of malignant bone and soft tissue tumors in conjunction with standard imaging techniques like MRI and CT scanning. The article also briefly touches upon the potential role of emerging PET-MRI modality.
Keywords: FDG-PET, Musculoskeletal tumors, Bone tumors, CT, MRI, Ewing Tumors, Osteosarcoma, GIST.
FDG PET and PET/CT
Positron emission tomography (PET) is a non-invasive nuclear imaging technique which relies on the detection of positrons emitted during the decay of a radionuclide and maps the biodistribution of the administered radiopharmaceutical. Compounds of interest are labelled with a positron-emitting radiotracer and infused and distributed according to the in vivo biologic behavior of the tagged compound. 18F-fluorodeoxyglucose (FDG) is the most commonly used PET radiopharmaceutical for oncology. FDG is a glucose analog in which the hydroxyl group is replaced by positron-emitting fluorine isotope (18F) and FDG-PET or FDG-PET/CT (when PET is combined with computed tomography) provides a map of glucose metabolism in the body. In contrast to anatomical and morphological approaches, FDG-PET provides more metabolic and functional information about the disease and can be an important imaging tool to non-invasively understand cancer biology [1]. FDG is actively taken up by cancer cells and remains metabolically trapped intracellularly. Otto Warburg, a German physiologist in the 1920’s, had shown that most tumor cells generate energy by non-oxidative breakdown of glucose and are hypermetabolic compared to the normal cells (The Warburg Effect). FDG-PET exploits this effect as cancer cells take up more FDG than normal cells and are hence detected on imaging as regions of increased FDG uptake. The concept of FDG-PET was developed in the 1970’s when it was used for functional brain imaging and then in the 1980’s to assess the cardiac metabolism. However, over the past 15 to 20 years, oncologic indications have become the predominant use for FDG-PET imaging and along with technological advances, it has now evolved to integrated PET/CT systems that provide highly sophisticated information with implementation of further hybrid imaging technologies, like combined PET/MRI, on the horizon [2].
With notable exceptions (such as prostate cancer), FDG PET/CT is routinely used for the initial treatment strategy (formerly encompassing diagnosis and staging) as well as a subsequent treatment strategy (formerly encompassing restaging and assessing treatment response as well as disease status) for most cancers, such as: lymphomas, lung cancer, colorectal cancer, melanomas, head and neck cancer, breast cancer, and musculoskeletal tumors and other malignancies. PET has largely been replaced by PET/CT scanners (at least in the Western nations) and this article will largely focus on PET/CT imaging instead of stand-alone PET imaging. Since MRI plays an important role in the evaluation of bone lesions, this article will briefly discuss the potential for combined PET/MRI hybrid imaging in the setting of bone and musculoskeletal tumors.
Musculoskeletal tumors
Malignant musculoskeletal tumors, also known as sarcomas, are rare and account for about 1% of cancer deaths in the United States [3]. They are a heterogeneous group of mesenchymal malignancies arising from bone and soft tissues. Primary bone tumors are seen more commonly in adolescents and younger adults, while primary soft tissue sarcomas are seen more commonly in adolescents with a second peak in the fifth decade. However, these sarcomas can affect all age groups. The World Health Organization’s classification of soft tissue sarcomas is based on the tissue of origin which continues to evolve with the discovery of new molecular genetic abnormalities [4]. The majority of soft tissue sarcomas are sporadic and only a few are linked to environmental factors like exposure to radiation, burns, toxins, viruses like HHV-8 causing Kaposi sarcoma in HIV patients, immunodeficiency syndromes, and germline mutations in Li-Fraumeni syndrome, neurofibromatosis 1, and Gardner syndrome. The common examples of soft tissue sarcomas include liposarcoma, synovial sarcoma, leiomyosarcoma (LMS), rhabdomyosarcoma (RMS), fibrosarcoma, and angiosarcoma. The patients usually present with an asymptomatic mass. The primary diagnosis is made by a tissue biopsy and imaging studies like plain radiograph, CT and MRI. Lungs are the most common site of metastases, and hence a plain radiograph and CT scan of the chest is also advisable. Treatment is based on AJCC staging. Stage IA (T1a-1b,N0,M0,G1,GX) and Stage IB (T2a-2b,N0,M0,G1,GX), low grade patients are usually managed by surgery by obtaining adequate oncologic margins. Stage IIA (T1a-b,N0,M0,G2,G3) can be managed with surgery alone, or surgery followed by radiotherapy or preoperative radiotherapy followed by surgery. Stage IIB (T2a-b,N0,M0,G2,G3) and Stage III (T2a,T2b,N0.M0,G3 and any T,N1,M0, Any G) if resectable with acceptable functional outcomes are managed with surgery followed by radiotherapy and adjuvant chemotherapy, or preoperative chemo-radiotherapy followed by surgery followed by adjuvant chemo-radiotherapy. Unresectable and resectable with adverse functional outcomes Stage II and III are managed with radiotherapy, chemotherapy, chemo-radiotherapy, or palliative surgery, alone or in combination. Synchronous Stage IV with single organ involvement or limited tumor bulk that are amenable to local therapy are managed primarily like Stage II and III tumors. Disseminated metastases are managed with palliative options. Accurate staging is critical for determining the appropriate treatment.
Gastrointestinal stromal tumors (GISTs) are discrete forms of sarcomas and are the most common abdominal mesenchymal tumors. They can arise anywhere in the gastrointestinal tract with the stomach being the most common site. Due to identification of driver mutations in the c-KIT and platelet-derived growth factor alpha genes encoding tyrosine kinase receptors, the treatment of GIST has been a role model of targeted therapy with Imatinib mesilate, a tyrosine kinase inhibitor [5, 6]. Surgery is still the main stay of management in resectable non-metastatic lesions with Imatinib playing an adjuvant role [7]. GISTs have variable clinical behavior with some presenting with nonspecific symptoms and some detected incidentally.
Bone sarcomas occur less commonly than soft tissue sarcomas and will account for 0.18% of all new malignancies, with 2970 estimated new cases and 1490 estimated deaths in the US in 2015 [3]. They are classified by Musculoskeletal Tumor Society Staging System based on grade and compartment localization. Osteosarcoma accounts for almost half of the bone sarcomas and is seen mainly in children and adolescent males in the metaphysis of long bones, especially the femur, the proximal tibia and the proximal humerus. Most of the cases are sporadic in nature with few cases arising from inherited genetic diseases like hereditary retinoblastoma and Li-Fraumeni syndrome. The patients usually present with pain and swelling of the affected area. Osteosarcomas are usually detected on imaging studies. The diagnosis is made by tissue sampling and pathology and can be suggested by imaging studies. These are usually high grade tumors with aggressive biological features and are found in or adjacent to areas with high bone growth, with subdetectable tumor spread elsewhere in majority of the cases [8, 9]. They are managed by neoadjuvant chemotherapy, which shrinks the tumor and targets micrometastatic tumor cells, followed by limb sparing surgery and adjuvant chemotherapy [9]. The prognosis is based on the response to chemotherapy. Radiation therapy generally has a limited role in the management of these tumors and is used mainly for unresectable and relapsed lesions [10]. Chondrosarcomas account for almost 25% of all bone sarcomas and are seen mainly in adult and old patients with predilection for flat bones. They have variable clinical behavior with an indolent nature and low metastatic potential [11]. Surgical resection is the standard of treatment. Radiation therapy is given in unresectable lesions. Chemotherapy is the primary therapy for systemic recurrence[10]. Ewing sarcoma constitutes approximately 10-15% of all bone sarcomas and is mainly seen in the second decade of life involving the diaphyseal region of the long bones, mostly in the lower extremity. These sarcomas present with localized pain or swelling of short duration. Constitutional symptoms are seen in small percentage of patients on presentation. They belong to a family of tumors known as PNETs (Primitive neuroectodermal tumors) and are associated with t(11;22) translocation[12]. The disease is aggressive and the presence of widespread metastasis is a sign of poor prognosis. It is primarily treated by multiagent chemotherapy and based on the response, is subsequently managed with radiotherapy, surgery or chemotherapy [10].
Improved diagnostic imaging has changed the primary management of musculoskeletal tumors. MRI is still the primary imaging technique used in detecting lesions and local staging due to its pluridirectional capabilities and superior contrast resolution. MRI thus plays an essential role in surgical planning by providing detailed information about the local extent of the disease and involvement of locoregional structures. MRIs are not, however, able to determine the subtypes of soft tissue sarcomas or differentiate between benign and malignant lesions. The regional nature of MRI also precludes identification of lymph nodes outside of the imaginary plane. Imaging distant metastatic disease is also not practical with routine MRI imaging studies. CT scans are not very sensitive for osseous pathology. Although CT has excellent spatial resolution, it is suboptimal to MRI when it comes to contrast resolution and soft tissue differentiation. CT scans are mainly used to assess pulmonary metastases and for staging of disease in the lungs in such patients [13]. Although used for assessing response to treatment based on shrinkage of the primary lesion, this approach may not be the best in the era of molecular imaging. Both CT and MRI have limitations in assessing local recurrence with altered anatomy and presence of post-therapy changes.
FDG PET/CT is not the optimal modality to assess the T-stage of these lesions. Although it can provide metabolic and functional information related to tumor biology, it has lower spatial resolution compared to morphologic imaging modalities and does not provide the intimate details about the local extent and invasiveness of the tumor. However, the intensity of FDG uptake can aid diagnosis by providing better targets for biopsy and increase the yield from biopsies. FDG PET imaging can also overcome some of the limitations of MRI, by separating high- from low-grade tumors, in determining the biological activity of a tumor, and by allowing the detection of abnormal lymph nodes and occult distant metastases, including pulmonary metastases, especially by virtue of almost whole body imaging [13]. However few studies have demonstrated that PET is less sensitive than CT scanning in the detection of pulmonary metastases and a significant number of known pulmonary metastases greater than 1.0 cm on CT, are PET negative (micro-metastases) [14]. Evolution of hybrid PET/MR may be a more efficient diagnostic modality in the future. It can provide additional information regarding soft-tissue analysis, tumor detection, tissue characterization, functional imaging and biological landscape at the same time.
Specific role of PET in musculoskeletal tumors
MRI and CT scanning are still the most commonly used imaging techniques to evaluate bone and soft tissue tumors with known limitations as discussed above. FDG-PET/CT imaging is now routine for cancer workup and the addition of a CT component in integrated PET/CT scanners have made this quite a reliable tool that can provide additional information about the biological behavior of the tumor and can aid in the management of these tumors.
Most soft and bone tumors are FDG-avid and the degree of avidity is usually associated with their clinical outcomes. In soft tissue sarcomas, FDG-PET is able to detect intermediate and high-grade lesions due to their high FDG uptake, but is not able to differentiate between benign and low-grade sarcomas since both of them tend to show low FDG uptake. Dual phase/delayed PET imaging can help in differentiating benign from malignant lesions in some cases as malignant lesions show increasing uptake on delayed images [15]. In bone tumors, low FDG uptake is usually seen in a benign lesion, with high FDG uptake in a malignant lesion. However, the highest FDG uptake is seen in metastases [16]. There are few exceptions to this rule; malignant tumors like plasmacytoma and low-grade chondrosarcoma can have low uptake, and benign tumors with either involvement of giant cells (giant cell tumor of bone) or histiocytic cells (Langerhans cells histiocytosis) can have high uptake. Using a TBR (tumor-to-background ratio) of 3.0 as a positive for malignant bone lesions, FDG PET has a specificity of 67% and a sensitivity of 93% in bone tumors [17]. The latest imaging guidelines set by Children’s Oncology Group Bone Tumor Committee highly recommend FDG-PET as a part of functional imaging in osteosarcoma and Ewing sarcomas at presentation and prior to surgery/local control. It also maintains use of FDG-PET for surveillance during and post chemotherapy [18].
Initial Treatment Strategy
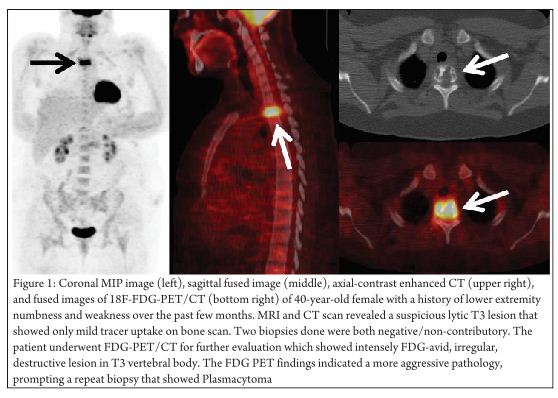
Diagnosis of musculoskeletal tumors is usually established on the basis of directed biopsies after the detection of a mass on clinical exam and/or imaging. As discussed above, they are staged per the AJCC system using the TNM staging criteria. Along with clinical evaluation, contrast enhanced CT and MRI are extremely useful for optimal assessment of the “T” stage as they provide further structural information regarding tumor extension and involvement of adjacent structures. FDG-PET imaging lacks the spatial resolution to provide such exquisite structural details necessary for adequate “T” staging. However, FDG-PET can still play a role in the diagnosis of these tumors. Many of these lesions can be heterogenous and initial biopsy can be “non-diagnostic”. (Figure 1 demonstrates the value of FDG-PET in a patient with a negative/non-contributory biopsy). Since FDG PET relies on the biologic characteristics of the tumor and provides metabolic and functional information, it can be suited in such difficult cases to direct biopsies to the appropriate target site and improve the yield from biopsies. In addition, it can play an important role in the detection of locoregional metastatic lymphadenopathy and distant metastatic disease. Traditional anatomical evaluation of nodal involvement in the malignancies is sub-optimal since nodes may be enlarged as a result of infection/inflammation (that is not uncommon in the groin region), and normal sized nodes can frequently be involved with metastatic disease leading to inaccurate upstaging or downstaging of the disease with conventional imaging methods. FDG-PET (and especially PET/CT) imaging can have a tremendous impact in improving the nodal staging of sarcomas cancers compared to CT/MR (sensitivity: 87-90% versus 61-90% and specificity: 80-93% versus 21-100%) [19]. FDG-PET imaging frequently detects metastatic disease in normal-sized lymph nodes. However, caution is recommended in N0 disease per PET as micrometastases cannot be detected by FDG-PET imaging and hence the management of such patients should not solely be determined by FDG-PET findings; other techniques like surgical lymph node dissection should be employed for optimal “N” staging in such patients. Also, sometimes malignant lymph nodes with large extensive central necrosis can be falsely negative on FDG-PET with only mild FDG uptake at the periphery or no uptake at all. However, the most important added value of FDG-PET imaging is the detection of unsuspected distant metastases that can lead to dramatic changes in patient management. By virtue of its near whole body imaging and reliance on metabolic information, it has the potential to detect unsuspected occult metastases and change the management significantly. Moreover, FDG PET imaging is useful in therapy planning for patients undergoing radiation therapy with a curative or palliative intent or as neoadjuvant therapy. The increasing implementation of intensity modulated radiation therapy (IMRT) is well complemented by the additional functional/metabolic information provided by the FDG imaging, as it allows delivery of maximal radiation dose to the most metabolically active areas of the tumor and more complete inclusion of loco-regional disease with sparing of the uninvolved areas.
Subsequent Treatment Strategy
In addition to the above, FDG-PET imaging probably has an important benefit in assessing response to therapy and restaging of musculoskeletal tumors [20-23]. Following surgery or radiation therapy, it is extremely difficult to assess the treated area with conventional imaging modalities like CT/MRI due to inflammatory changes with fibrosis, edema and alteration of normal structures. Determining whether residual neoplasm is present in the postsurgical/postradiated tumor bed is one of the most daunting tasks facing radiologists. When compared to conventional radiological examination, FDG-PET has a better diagnostic accuracy in the assessment of residual or recurrent malignant disease in the post-therapeutic region, including avoidance of unnecessary planned surgery in patients with negative PET. Lack of any significant FDG uptake in the treated area generally indicates no active residual/recurrent disease. There may be some mild to modest irregular FDG uptake related to post-therapy changes, but generally there should be no gross intense focal abnormalities. Dual-phase PET imaging/delayed PET imaging may help in distinguishing post-therapeutic inflammatory changes from cancerous tissue. It may also help in the prediction of PFS (Progression free survival) and OS (overall survival). Focal intense FDG uptake within the area of post-surgical change is worrisome and needs further workup. A negative tissue biopsy after a strongly positive post-treatment PET scan can be caused by sampling error and warrants a closer follow-up rather than routine surveillance. Decrease in the intensity of uptake on the follow-up scan confirms a false positive post-treatment PET scan, usually due to inflammatory changes. However, persistence of a focally intense lesion or increase in the intensity of uptake warrants invasive evaluation. The timing of the post-treatment PET scan is very crucial, especially after radiation therapy. Although there are no specific recommendations in this regards, generally a 3-month interval after completing radiation therapy is felt to be adequate to assess response to therapy. The superior assessment of response to therapy with FDG-PET imaging may facilitate a more conservative approach in management, as patients undergoing combined chemo-radiation therapy with a complete response on the post-treatment FDG-PET scan can be followed with a more watchful approach.
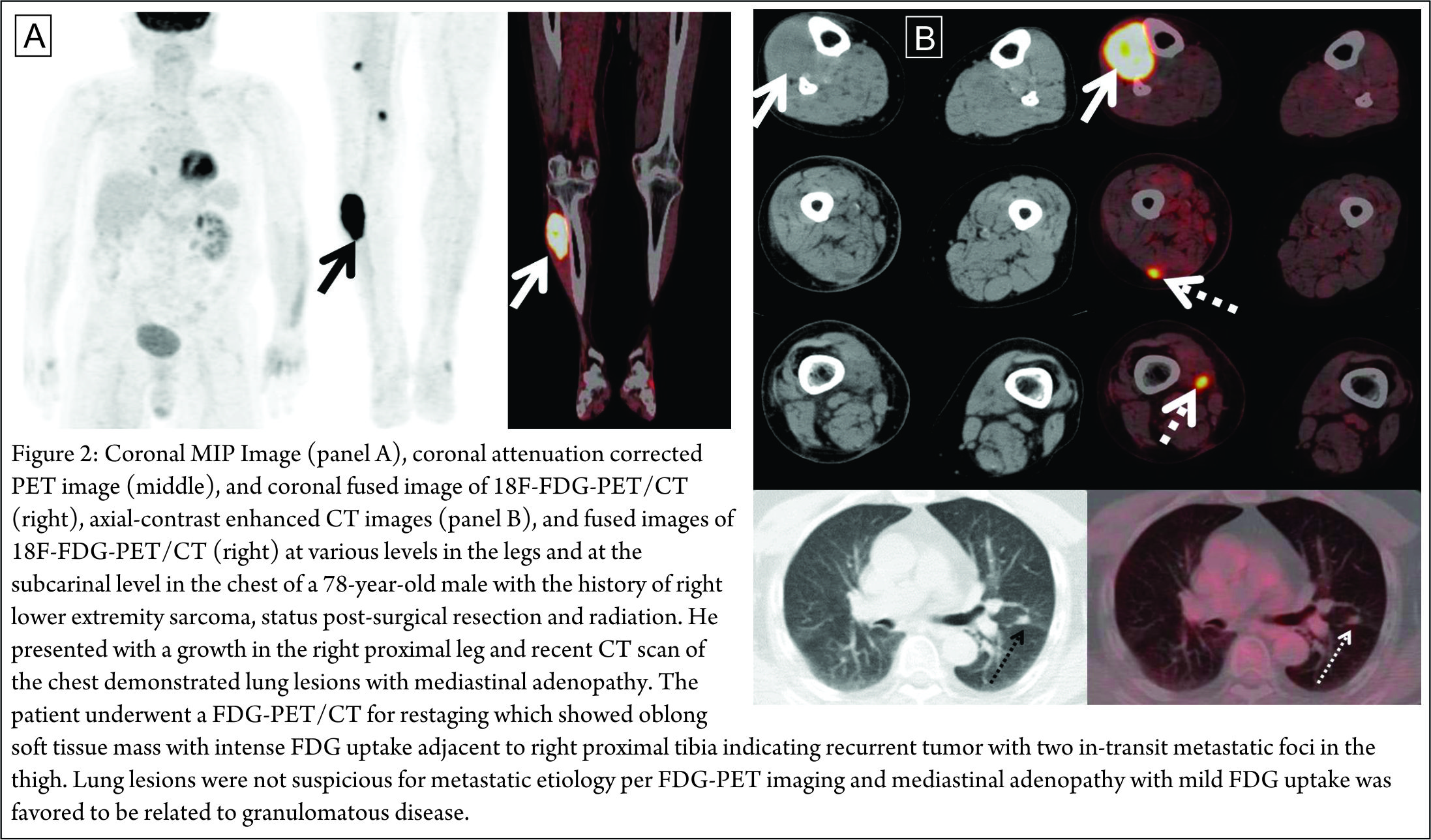
There are several limitations of FDG-PET imaging in the evaluation of musculoskeletal tumors. Although it may detect tumors that may be missed by anatomic imaging (especially in-transit metastases as in Figure 2), the sub-optimal spatial resolution of PET imaging (compared to CT/MRI) limits the evaluation of local extent and invasiveness of the tumor. Also, low-grade tumors may be missed on PET if there is significant intense physiologic FDG uptake in an adjacent structure (like muscle). Conditions like joint inflammation, muscle contraction, radiation induced inflammation and osteoradionecrosis need to be kept in mind when interpreting FDG-PET studies in musculoskeletal pathology. The added information from CT images in a dedicated PET/CT scan can further help to discern this uptake as benign/physiologic.
Osteosarcoma
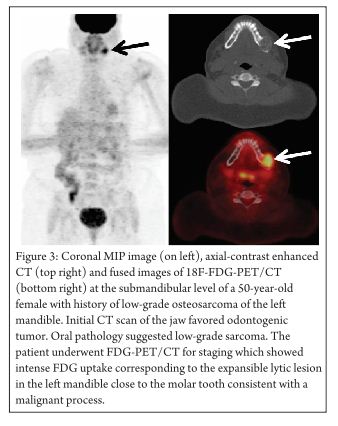
After the advent of neo-adjuvant chemotherapy in osteosarcoma, which has dramatically improved the prognosis, there has been a need for better imaging modality for tumor staging and grading, pre- and post-treatment evaluation, and detection of tumor recurrence (Figure 3 demonstrates FDG uptake may be quite heterogeneous and intense in osteosarcoma).
Initial Treatment Strategy
FDG-PET/CT imaging has a limited role in the initial workup of osteosarcoma. It is limited in its ability to diagnose osteosarcoma (which definitely requires tissue sampling) and is suboptimal to CT/MRI in delineating the local extent and invasiveness. The correlation between the histological grading and the FDG avidity has been well documented by several studies [24]. However, FDG-PET/CT imaging cannot obviate the need for the tumor biopsy to differentiate between a benign and a malignant lesion and establish the underlying pathology. The highest SUV values are seen in bone metastases. MRI and plain radiographs are still the first line diagnostic tools in staging the disease. In children, there may be an indication of FDG-PET in cases of unequivocal MRI findings due to physiological red blood marrow distribution to detect interosseous skip metastases. Lymph node metastasis is a rare phenomenon in osteosarcoma and hence the need of PET is limited. About 80% of metastases in osteosarcoma involve the lungs and early detection is important. The method of choice for detecting lung metastases is spiral high-resolution CT as PET can miss smaller lung lesions [25] However, whole body imaging in PET has an advantage of finding other sites of occult metastases, which cannot be seen with CT or MRI due to limited field of scanning and so should be employed in situations where clinical suspicion for metastatic disease is high. Infrequently, it may be used to guide biopsies if clinically necessary.
SUVmax and TLG (Total lesion glycolysis) are both strong prognostic factors that can predict progression-free survival, overall survival, and tumor necrosis in osteosarcoma [26].
Subsequent Treatment Strategy
FDG-PET plays a more established role in assessing therapy response and detecting recurrence. It has also been able to predict the tumor response as it relies on functional and metabolic parameters rather than structural changes. Tumor metabolic changes detected by FDG-PET precede morphological changes on anatomic imaging and early evaluation of tumor response allows treatment to be tailored to the individual. In two different studies, FDG-PET was found to be superior to MRI in the assessment of response [20, 21]. There is a direct correlation between SUV and histological grade. SUVmax reduction after therapy is the biggest indicator of whether the patient is responding to therapy or not, and based on this, the therapy can be modulated accordingly. SUVmax > 5 after neoadjuvant therapy is arbitrarily defined as a histological nonresponder and ≤ 2 as a responder [20-22, 27]. Byun et al suggested that the combination of FDG-PET/CT and MRI may be the best way to determine histological response of osteosarcoma after neoadjuvant chemotherapy [23]. The availability of combined PET/MRI imaging in the future may facilitate this.
FDG-PET has also a significant role in the assessment of tumor recurrence and restaging of high risk osteosarcoma patients. (28) It is also more accurate than other imaging studies in differentiating post-therapeutic fibrosis or inflammatory changes from local recurrence [25].
Chondrosarcomas
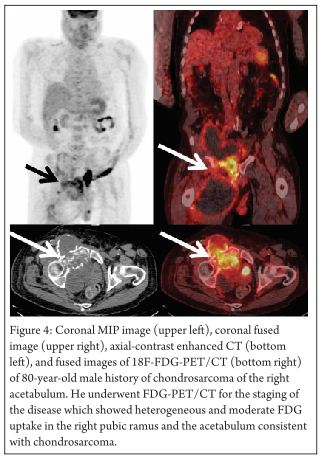
These sarcomas have less FDG uptake than other sarcomas owing to their high level of acellular gelatinous matrix and lower mitotic rates. Average FDG uptake of chondrosarcoma is as high as Ewing sarcoma but lower than osteosarcoma [29]. The role of PET in the diagnosis and management of chondrosarcoma is almost the same as with other malignant bone lesions. The biological activity helps to assess the tumor grade and to differentiate between benign and malignant tissue, and the whole body imaging helps to identify any occult metastases. Grade II and III chondrosarcomas have higher glucose metabolism and can be easily distinguished from a benign tumor; Grade I chondrosarcomas/atypical cartilaginous tumors cannot be so easily distinguished because of apparently similar metabolism rates [30]. (Figure 4 demonstrates the heterogeneous nature of FDG uptake in chondrosarcoma; intense FDG uptake site can help in guiding the biopsy in such patients)
Ewing sarcomas
Ewing sarcomas are high-grade malignancies and high SUVs are usually seen. PET is very sensitive in the detection of primary and recurrent lesions. PET is also superior to bone scan in detecting bone metastases and is used as a part of metastatic workup. PET has low sensitivity for smaller lesions, especially in lungs which are a common site of metastases for Ewing sarcomas and a CT scan is a superior imaging modality in such cases. PET can also be used for monitoring the tumor response to chemotherapy and radiotherapy and the possibility of a recurrence post-operatively. PET has a limitation in differentiating malignant from inflammatory lesions and cannot be used as a non-invasive diagnostic tool between Ewing sarcoma and osteomyelitis, which are frequently indistinguishable [31].
Fibrosarcoma
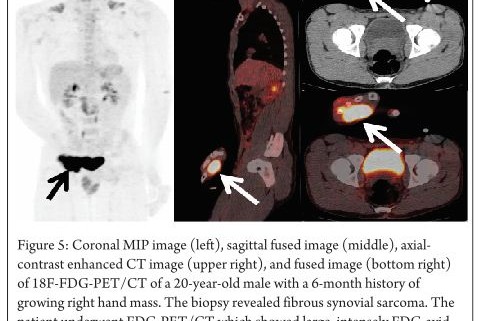
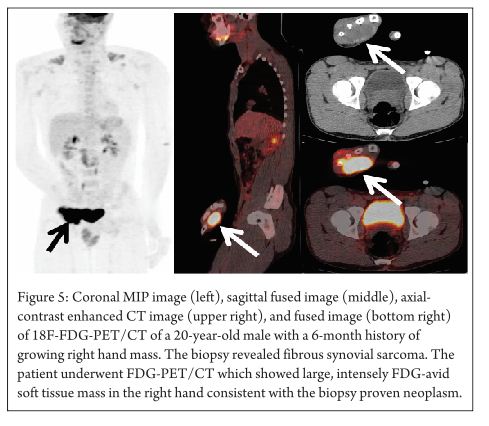
Fibrosarcomas arising from polyostotic fibrous dysplasia have intense FDG uptake indicating sarcomatous transformation. Fibrous dysplasia sarcomas are well known to have intense FDG uptake despite their benign nature [32]. Fibrous synovial sarcomas originate from the mesenchymal tissue and their histological appearance resembles the synovium. FDG-PET can also be used for the staging of these malignant tumors. (Figure 5 demonstrates intensely FDG avid soft tissue mass)
Gastrointestinal stromal tumors
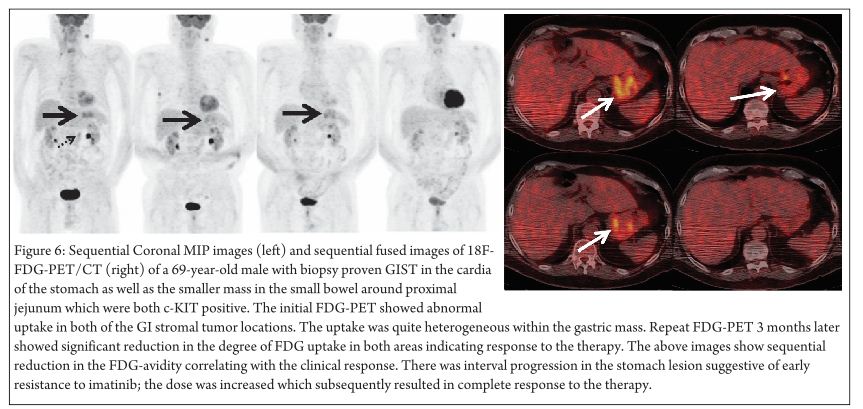
Metabolic imaging with FDG-PET in GIST has proven to be an effective tool to evaluate the treatment response with tyrosine kinase inhibitors like imatinib. The functional imaging with FDG-PET provides earlier evidence of response in comparison to morphological changes seen with a CT scan. Jager et al observed that changes in tumor metabolism were seen as early as 1 week after the start of the treatment, which helped in delineating responders from non-responders in 14/15 cases [33]. Studies done by Stroobants et al and Goerres et al showed that PET responders had a better progression free survival and better prognosis than PET non-responders with residual FDG activity.(34, 35) However a recent study done by Chacon et al showed the early metabolic response (EMR) does not correlate with the progression-free survival or overall survival in patients with metastatic GIST. (36) GIST-specific molecular tracers are also in the making which can provide more accurate prognosis and development of treatment resistance. (37) FDG negativity however does not preclude the diagnosis of a GIST [38] (Figure 6 demonstrates the value of FDG-PET as a prognostic tool in the management of GIST)
Benign Tumors
FDG-PET has a limited role in the management of benign musculoskeletal tumors. Benign soft tissue lesions usually do not have substantial FDG uptake. Fibrous dysplasia can have variable FDG uptake, and in some cases intense FDG activity. In such situations, it is important to differentiate benign tumors from any possibility of a sarcomatous changes [39]. Hemangiomas can also be a site of intense FDG activity which can sometimes mimic metastasis. Lipomas have the lowest uptake. Careful history, physical examinations and other imaging tests like CT and MRI should help in the accurate diagnosis.
Conclusion
The evolution of PET in the recent years has changed the previous paradigm in the management of malignancies. In general, it is not the primary diagnostic modality for workup of musculoskeletal tumors but can play a role in certain clinical scenarios. Along with other imaging techniques, FDG PET/CT plays an important role in musculoskeletal tumors by guiding biopsies in heterogeneous tumors, predicting tumor response to preoperative neo-adjuvant chemotherapy, detecting skip metastases and reflecting risk of recurrence and prognosis. It also plays a more robust role in subsequent treatment strategy. Overall, it is more useful in evaluating primary soft tissue tumors relative to primary osseous lesions. However, the potential availability of integrated PET/MRI may allow for a more robust role for FDG-PET imaging in the workup of primary osseous tumors as well. FDG-avidity correlates negatively with survival and positively with disease progression. It can be used to tailor treatment, surgical versus chemo-radiotherapy. More prospective trials are needed to develop new tracers that can be more specific and lead to higher signal to noise ratio (SNR), which may help in establishing the response to treatment with newer agents and can set guidelines. Suboptimal T-stage and heterogeneous uptake in some cases, insufficient topography, radiation exposure and higher costs are a few of the limitations of using FDG-PET. In the current times, its role is still considered as an adjunct and has not replaced MRI and CT scanning. The combined PET-MRI multimodality imaging systems can provide adequate information about the morphology as well as the metabolic status of the lesion in a single imaging session and may potentially become the standard of imaging for musculoskeletal tumors in the near future. Precision medicine (prevention and treatment strategies that take individual variability into account) is the way to the future. Adopting global disease assessment, radiotherapy fractionation, imaging hypoxia, adaptive radiotherapy as part of quantifiable methodologies and standardization of FDG-PET, it can become a powerful tool for the diagnosis, individual treatment planning and subsequent treatment strategy. The absolute potential of FDG-PET in various malignancies including musculoskeletal tumors is still a work in progress and is evolving at a rapid pace with the recent development of radiopharmaceuticals and technological advancements..
References
1. Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2(9):683-93.
2. Takalkar AM, El-Haddad G, DL L. FDG-PET AND PET/CT – Part I. Indian Journal of Radiological Imaging. 2007;17(3):169-80.
3. American Cancer Society. Cancer Facts and Figures 2015. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf.
4. Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46(2):95-104.
5. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-80.
6. Cassier PA, Dufresne A, Arifi S, El Sayadi H, Labidi I, Ray-Coquard I, et al. Imatinib mesilate for the treatment of gastrointestinal stromal tumour. Expert Opin Pharmacother. 2008;9(7):1211-22.
7. NCCN. Soft tissue sarcoma version 1.2015 [April 28, 2015]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf.
8. Heare T, Hensley MA, Dell’Orfano S. Bone tumors: osteosarcoma and Ewing’s sarcoma. Curr Opin Pediatr. 2009;21(3):365-72.
9. Bruland OS, Høifødt H, Saeter G, Smeland S, Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res. 2005;11(13):4666-73.
10. NCCN. Bone Cancer Version 1.2015 [April 4, 2015]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/bone.pdf.
11. Pring ME, Weber KL, Unni KK, Sim FH. Chondrosarcoma of the pelvis. A review of sixty-four cases. J Bone Joint Surg Am. 2001;83-A(11):1630-42.
12. Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing’s sarcoma: standard and experimental treatment options. Curr Treat Options Oncol. 2009;10(1-2):126-40.
13. Borden EC, Baker LH, Bell RS, Bramwell V, Demetri GD, Eisenberg BL, et al. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res. 2003;9(6):1941-56.
14. Iagaru A, Chawla S, Menendez L, Conti PS. 18F-FDG PET and PET/CT for detection of pulmonary metastases from musculoskeletal sarcomas. Nucl Med Commun. 2006;27(10):795-802.
15. Tian R, Su M, Tian Y, Li F, Li L, Kuang A, et al. Dual-time point PET/CT with F-18 FDG for the differentiation of malignant and benign bone lesions. Skeletal Radiol. 2009;38(5):451-8.
16. Watanabe H, Shinozaki T, Yanagawa T, Aoki J, Tokunaga M, Inoue T, et al. Glucose metabolic analysis of musculoskeletal tumours using 18fluorine-FDG PET as an aid to preoperative planning. J Bone Joint Surg Br. 2000;82(5):760-7.
17. Schulte M, Brecht-Krauss D, Heymer B, Guhlmann A, Hartwig E, Sarkar MR, et al. Grading of tumors and tumorlike lesions of bone: evaluation by FDG PET. J Nucl Med. 2000;41(10):1695-701.
18. Meyer JS, Nadel HR, Marina N, Womer RB, Brown KL, Eary JF, et al. Imaging guidelines for children with Ewing sarcoma and osteosarcoma: a report from the Children’s Oncology Group Bone Tumor Committee. Pediatr Blood Cancer. 2008;51(2):163-70.
19. Mak D, Corry J, Lau E, Rischin D, Hicks RJ. Role of FDG-PET/CT in staging and follow-up of head and neck squamous cell carcinoma. Q J Nucl Med Mol Imaging. 2011;55(5):487-99.
20. Kong CB, Byun BH, Lim I, Choi CW, Lim SM, Song WS, et al. ¹⁸F-FDG PET SUVmax as an indicator of histopathologic response after neoadjuvant chemotherapy in extremity osteosarcoma. Eur J Nucl Med Mol Imaging. 2013;40(5):728-36.
21. Cheon GJ, Kim MS, Lee JA, Lee SY, Cho WH, Song WS, et al. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med. 2009;50(9):1435-40.
22. Byun BH, Kim SH, Lim SM, Lim I, Kong CB, Song WS, et al. Prediction of response to neoadjuvant chemotherapy in osteosarcoma using dual-phase (18) F-FDG PET/CT. Eur Radiol. 2015.
23. Byun BH, Kong CB, Lim I, Choi CW, Song WS, Cho WH, et al. Combination of 18F-FDG PET/CT and diffusion-weighted MR imaging as a predictor of histologic response to neoadjuvant chemotherapy: preliminary results in osteosarcoma. J Nucl Med. 2013;54(7):1053-9.
24. Rakheja R, Makis W, Skamene S, Nahal A, Brimo F, Azoulay L, et al. Correlating metabolic activity on 18F-FDG PET/CT with histopathologic characteristics of osseous and soft-tissue sarcomas: a retrospective review of 136 patients. AJR Am J Roentgenol. 2012;198(6):1409-16.
25. Brenner W, Bohuslavizki KH, Eary JF. PET imaging of osteosarcoma. J Nucl Med. 2003;44(6):930-42.
26. Costelloe CM, Macapinlac HA, Madewell JE, Fitzgerald NE, Mawlawi OR, Rohren EM, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50(3):340-7.
27. Denecke T, Hundsdörfer P, Misch D, Steffen IG, Schönberger S, Furth C, et al. Assessment of histological response of paediatric bone sarcomas using FDG PET in comparison to morphological volume measurement and standardized MRI parameters. Eur J Nucl Med Mol Imaging. 2010;37(10):1842-53.
28. Peller PJ. Role of positron emission tomography/computed tomography in bone malignancies. Radiol Clin North Am. 2013;51(5):845-64.
29. Costelloe CM, Chuang HH, Chasen BA, Pan T, Fox PS, Bassett RL, et al. Bone Windows for Distinguishing Malignant from Benign Primary Bone Tumors on FDG PET/CT. J Cancer. 2013;4(7):524-30.
30. Lee FY, Yu J, Chang SS, Fawwaz R, Parisien MV. Diagnostic value and limitations of fluorine-18 fluorodeoxyglucose positron emission tomography for cartilaginous tumors of bone. J Bone Joint Surg Am. 2004;86-A(12):2677-85.
31. Györke T, Zajic T, Lange A, Schäfer O, Moser E, Makó E, et al. Impact of FDG PET for staging of Ewing sarcomas and primitive neuroectodermal tumours. Nucl Med Commun. 2006;27(1):17-24.
32. Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of (18)F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med. 2008;22(7):603-9.
33. Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumours: best monitored with FDG PET. Nucl Med Commun. 2004;25(5):433-8.
34. Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer. 2003;39(14):2012-20.
35. Goerres GW, Stupp R, Barghouth G, Hany TF, Pestalozzi B, Dizendorf E, et al. The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumours: long-term outcome of treatment with imatinib mesylate. Eur J Nucl Med Mol Imaging. 2005;32(2):153-62.
36. Chacon M, Eleta M, Rodriguez Espindola A, Roca E, Mendez G, Rojo S, et al. Assessment of early response to imatinib 800 mg after 400 mg progression by F-18-fluorodeoxyglucose PET in patients with metastatic gastrointestinal stromal tumors. Future Oncology. 2015;11(6):953-64.
37. Ronellenfitsch U, Waengler B, Niedermoser S, Dimitrakopoulou-Strauss A, Hohenberger P. Importance of PET for surgery of gastrointestinal stromal tumors. Chirurg. 2014;85(6):493-9.
38. Williams A, Gutzeit A, Germer M, Pless M. PET-Negative Gastrointestinal Stromal Tumors. Case Rep Oncol. 2013;6(3):508-13.
39. Santiago Chinchilla A, Ramos Font C, Tello Moreno M, Rebollo Aguirre AC, Navarro-Pelayo Láinez M, Gallego Peinado M, et al. [Fibrous dysplasia of the bone. Contribution of nuclear medicine in the diagnosis of suspicion of sarcomatous degeneration]. Rev Esp Med Nucl. 2010;29(4):172-6.
| How to Cite this article: Ansari J, Munker R, Takalkar A. Current role of FDG-PET in Bone and Soft tissue tumors. Journal of Bone and Soft Tissue Tumors May-Aug 2015; 1(1):29-36. |