Volume 2 | Issue 2 | May-Aug 2016 | Page 20-24 | Vincent S Paramanandam, Anuradha A Daptardar, Ashish Gulia
Authors: Vincent S Paramanandam [1], Anuradha A Daptardar [1], Ashish Gulia [2].
1Physiotherapy Department, Tata Memorial Hospital, Mumbai
2Orthopedic Oncology Services, Department of Surgical Oncology, Tata Memorial Hospital, Mumbai.
Address of Correspondence
Dr. Paramanandam V
Technichal Officer C, Physiotherapy Department, Tata Memorial Hospital, Mumbai
Email: vinsu24@gmail.com
Abstract
Introduction: Limb salvage after tumor resection has become a norm in today’s era. There are number of biological and non biological reconstruction options available for the reconstruction of these bone defects. The success story of these surgical procedures is mainly based on their excellent functional outcome. Post surgical rehabilitation plays an important role in achieving optimal functional outcome and good quality of life. The rehabilitation protocol following limb salvage surgery is complex and it differs with type of reconstruction procedure. Present articles discusses in detail the various rehabilitation protocols required to achieve above goals.
Keywords: Limb salvage surgery, rehabilitation, sarcoma
Introduction
Until 1970, amputation was the primary surgical treatment offered to bone and soft tissue sarcomas. However, from that time the treatment options have evolved dramatically and now approximately 90% of these cases undergo Limb Salvage Surgery (LSS)[1]. LSS has become the main line of treatment option for bone and soft tissue sarcomas along with adjuvant and/or neo adjuvant treatment modalities (Chemotherapy/ radiotherapy). The overall survival rate has been estimated as 55%-65%, based on the age of diagnosis, and it is considered to be comparable to that of amputation.
LSS is considered to be less invasive, provides better function and quality of life than amputation [2]. Moreover, it has been proposed that patients’ acceptability of LSS is high in view of the fact that it restores the body image better than amputation[3]. Nevertheless, LSS, unlike amputation, is associated with more peri-operative complications, prolonged hospital stay and requires repeated surgeries due to various reasons such as infection and prosthetic failure. LSS demands high surgical skills, whereas, amputation is a simple surgical procedure. Additionally, recent progress in prosthetic limbs, for example microprocessor based joints and endo-skeletal prosthetic reconstructions, have improved the functional outcome and cosmetic outlook following amputation [4]. A systematic review conducted by Bekkering et al [5] reported that the quality of life outcome from current available evidence is inconclusive in supporting LSS or amputation. Another recent systematic review and meta-analysis concluded that both surgical procedures provides similar functional recovery and quality of life [6].Despite the fact that early physical rehabilitation is the key to achieve good functional outcome and quality of life after LSS, rehabilitation techniques following LSS is largely neither tested nor documented in detail [7]. Lack of adequate early rehabilitation measures following LSS could be one of the rationales for conflicting interests reported by various studies examining the quality of life in LSS vs amputation.Hence, we have attempted to summarise basic principles and site specific considerations one must utilise to develop individual case specific rehabilitation protocol.
Common rehabilitation principles in LSS
In a recent paper, Shehadeh et al. [7] attempted to standardize the rehabilitation protocol for LSS following high grade bone and soft tissue sarcomas. They reported that following a standardized rehabilitation protocol produced improved functional outcome in group of 59 patients with LSS. Their conclusion, however, is based on small observational study with heterogeneous population who received different type of LSS for different anatomical sites. Following set protocol in LSS, unlike general orthopaedic procedure, will be counterproductive. In general orthopaedic procedures, more or less,
specific anatomical structures are involved with minimal damage to the bone, joint and soft tissue structures. In contrast, in LSS following sarcomas, these structures are extensively resected and may not be identical between two individuals undergoing similar procedures for a particular site. For example, resection length for distal end of femur osteogenic sarcoma may depend on the extent of disease in two individuals [4]. Following are common rehabilitation prospectives that need to be considered to formulate comprehensive rehabilitation protocol for LSS. 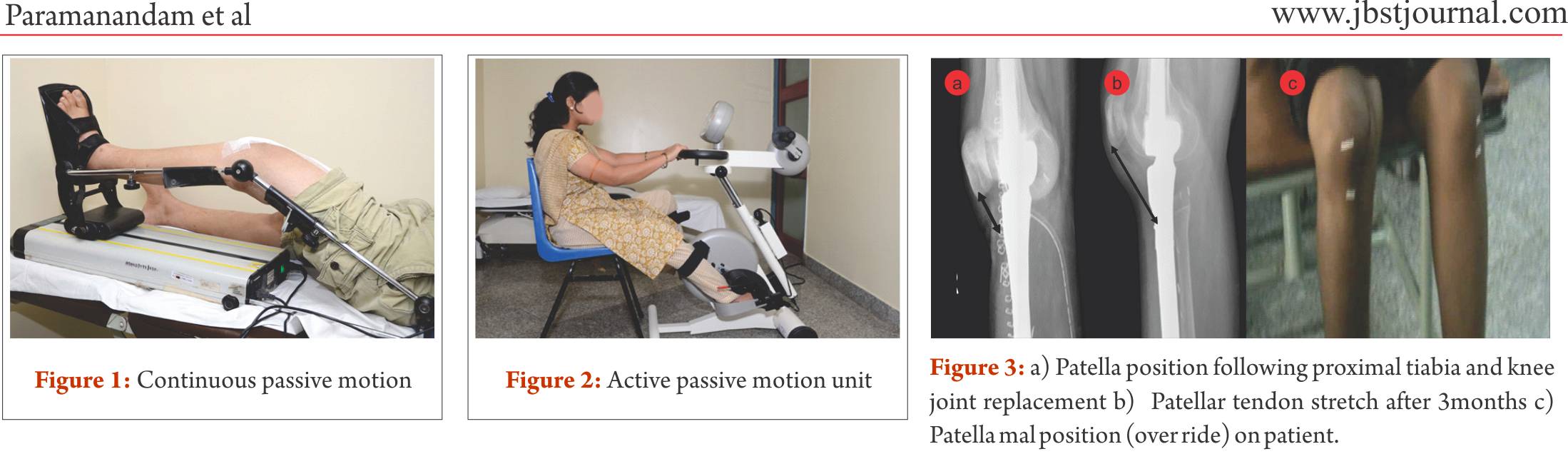
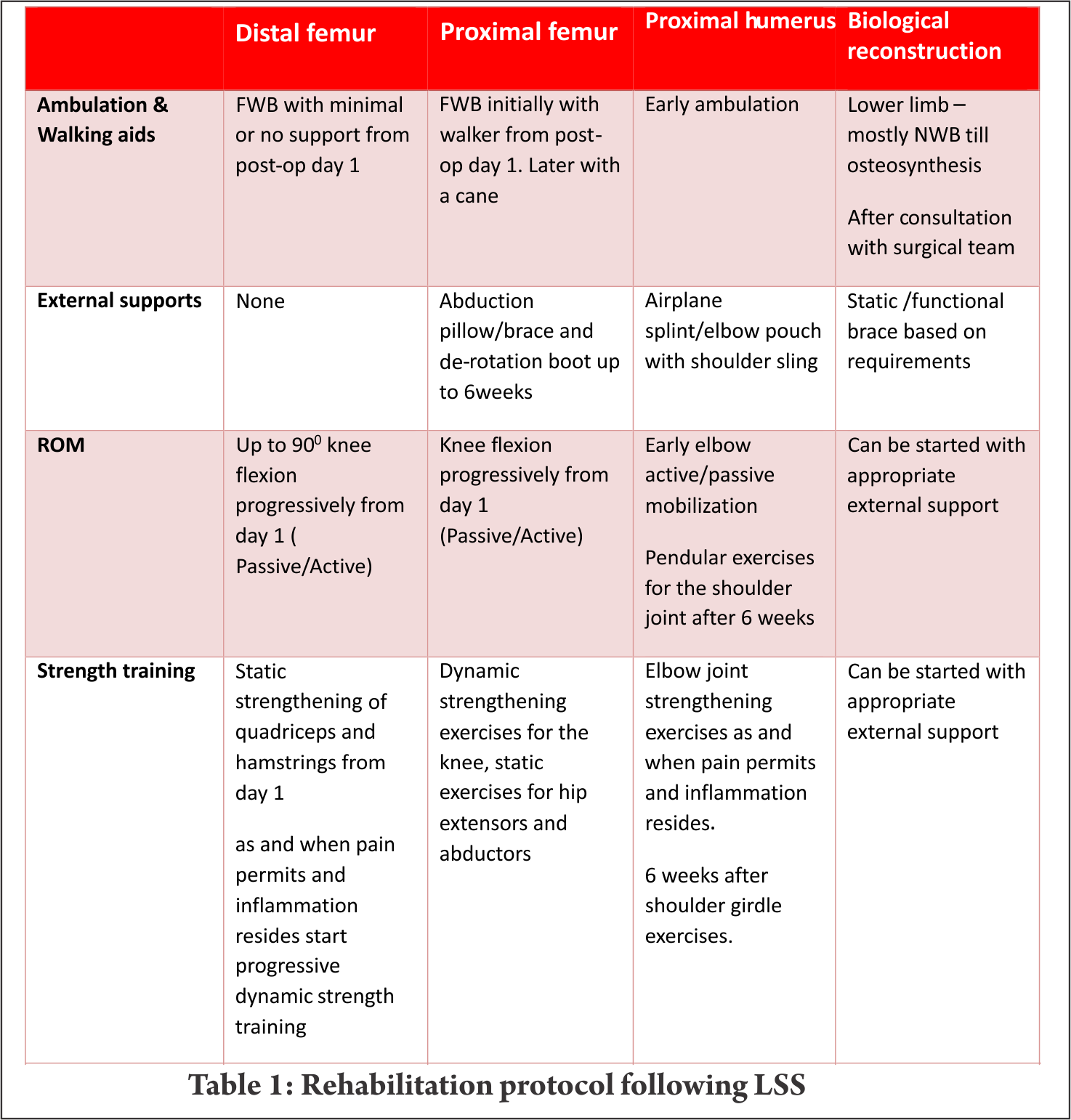
Bone and joint reconstructions: Stability and mobility following LSS largely depends on the bone and joint structure loss and the type of reconstruction. For example, megaprosthetic distal femur replacement with cementing will allow the patient to be ambulated full weight bearing (FWB), whereas, if it is a bone graft , like in most of biological reconstructions, weight bearing needs to be delayed till the osteosynthesis is confirmed by radiographical evaluation.
Neuromuscular loss: Oncology resection demands large resections, which will also include a part of uninvolved soft tissue cover as surgical margin. Large resection may require additional rotational or free flaps for soft tissue coverage. In addition, nearby neuro-vascular bundle may need to be excised or repaired, hence, complete evaluation of neuro-motor loss would be necessary to plan the dynamic strength training and external support requirements.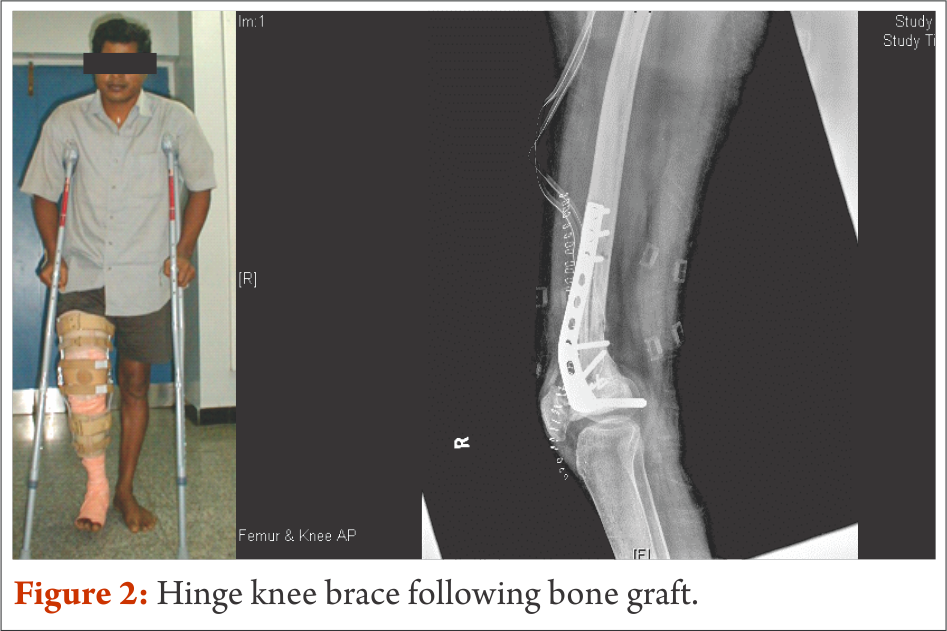
Skin involvement: Donor sites of free flaps often receive split thickness skin graft which may hinder the early mobilisation of nearby joint. Moreover, scar development following open biopsy and LSS may need special attention from the rehabilitation team to prevent any future functional loss.
External supports: Temporary or permanent external support in the form of static or dynamic splinting may be required to provide support to the limb. To exemplify, prophylactic use of abduction brace along with derotation boot to prevent hip dislocation following proximal femur replacement and dynamic cock-up splint for radial nerve palsy needs to be the integral part of rehabilitation service.
Oncology treatments: Deranged blood count often hinders with the intensity of rehabilitation; hence, it prolongs the overall rehabilitation(8). Radiation induced fibrosis could cause severe restrictions in the joint range of motion. Thus, rehabilitation professionals must plan around the chemotherapy cycles and add prophylactic measures to prevent any impending radiation induced joint and soft tissue dysfunction.
Multidisciplinary approach: Limb salvage surgery is complex and demands close concordance in treatment specific outcomes between various health professional working in the rehabilitation team. This team may comprise of surgeons, medical oncologists, radiation therapists, nurses, physiotherapists, occupational therapists, prosthetics orthotics and medical social workers. Prehabilitation, rehabilitation even before starting the primary cancer therapy and surgery, such as crutch muscles strengthening, would be greatly beneficial in post-treatment functional outcome. Although in the field of LSS evidence of prehabilitation is lacking, there are considerable evidence to show beneficial effects in overall rehabilitation following cancer therapies [9–11].
Rehabilitation prescriptions and follow-up: Rehabilitation protocol for LSS must be tailor made considering the general principles and site specific modification, hence specific and also progressive. However, some negative effects of adjuvant therapies, such as the deranged blood counts and infection, may alter the course of rehabilitation process. Thus, frequent follow-up and close monitoring may be required during adjuvant therapy till they are functionally independent.
Rehabilitation consideration for specific sites
Site specific rehabilitation principles following LSS have been presented below for few common sites.
Mega prosthetic replacement for distal femoral resection:
Distal femur is the commonest site for primary high grade sarcoma and giant cell tumors. Overall strengthening other than the affected site, in all possibility, should begin preoperatively. Limb elevation and ankle toe movement should be encouraged from post operative day one to prevent deep vein thrombosis. Cemented and semiconstrained (allows rotations and flexion/extension) knee joint endoprosthetic replacement permits early joint mobilization and FWB walking. Unlike other centres [4,7], in our centre knee joint mobilisation starts from day one with the help of continuous passive motion units (Fig.1) and active assisted methods within the pain tolerance level unless tight suturing. Close communication between the surgical team and the rehabilitation team helps in personalising the rehabilitation protocol as per the patients’ requirements. With adequate pain relief through appropriate medical management, active exercises could be started from day one to three. Full weight bearing walking could be started from day one initially with walker and later without any support if patient could effectively extend the knee “locking the knee”. Prior to ambulation, one leg standing and spot marching must be encouraged with appropriate support. After acute inflammation subsides slow progressive muscle strengthening exercises must be encouraged with the goal of achieving 900knee flexion, complete knee extension and muscle strength equivalent to the contra lateral lower limb by end of three months. Active passive motion devices, such as the one shown in Fig. 2, may help in joint mobilisation and strengthening. Summary of rehabilitation protocol is tabulated in Table 1.
Mega prosthetic replacement for proximal tibial resection:
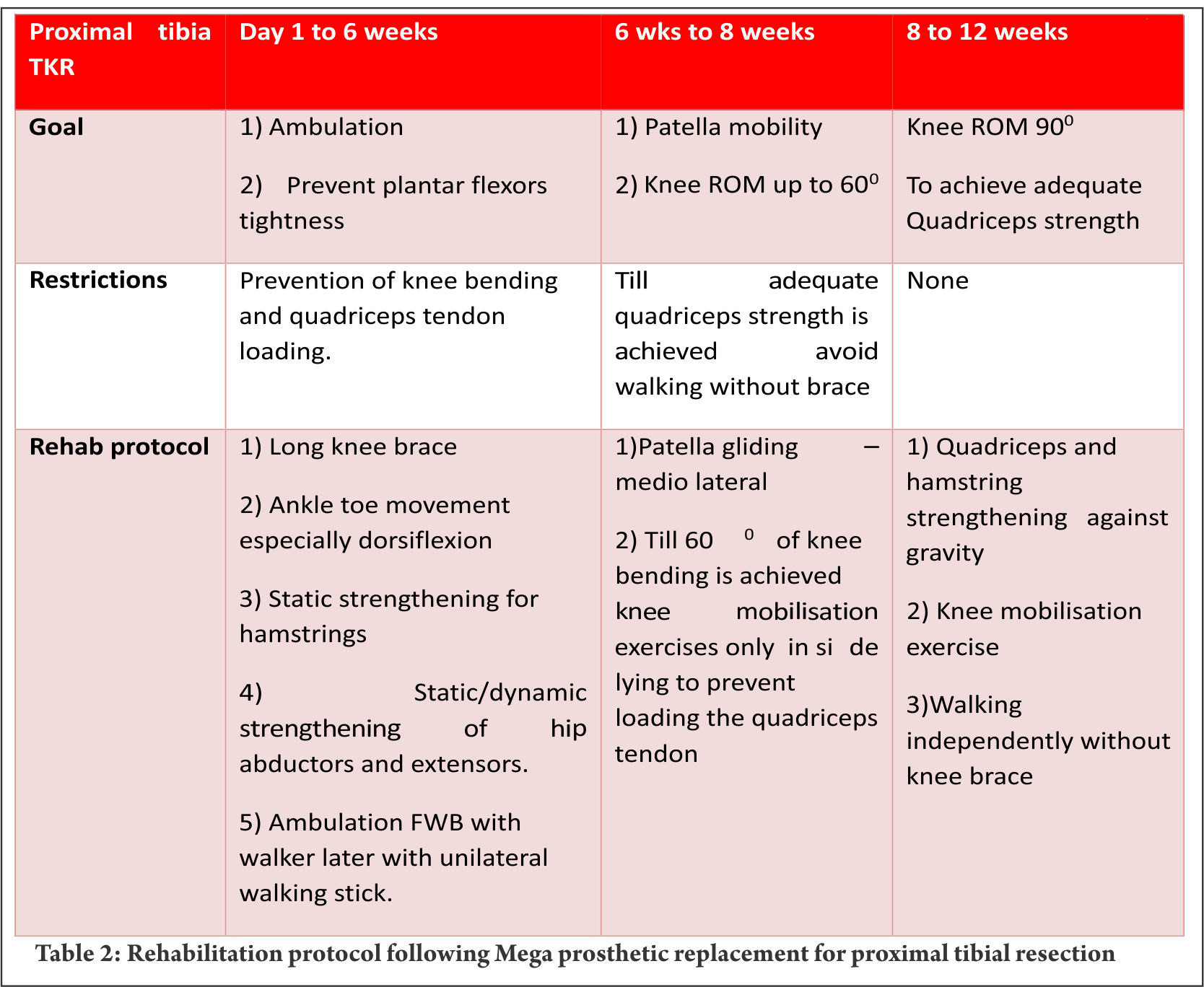
Proximal tibia and knee joint is the second most common site for primary high grade sarcoma and giant cell tumors. It is indeed a challenging location for rehabilitation in view of the fact that the extensor mechanism have to be reconstructed in these cases [4]. In most of the cases a gastrocnemius flap is done to provide a dynamic anchorage and direct anchorage to prosthesis provide a static attachment to help reattaching extensor mechanism to proximal tibial prosthesis. Protecting the extensor mechanism reconstruction till it attaches through fibrosis along with the surrounding soft tissue structures are crucial to prevent quadriceps lag. Hence, knee bending and quadriceps strengthening will be delayed for six weeks until then the knee is protected with the help of long knee brace. Re-attached gastrocnemius flap could lead to protective muscle spasm of plantar flexors and if not mobilised early it may create plantar flexors contracture. Thus, achieving/maintaining dorsi flexion of the ankle joint in the early post operative period is crucial for appropriate weight bearing. Mobilisation of knee joint and quadriceps strengthening could be started after six weeks; however, therapist must consider that immobilisation of the knee joint in long knee brace leads to severe restriction of patella mobility. Unless adequate patella mobility is achieved, especially the medial lateral movement, knee flexion exercises could prevent smooth gliding of patella over the femoral condyle. This will increase strain on the reconstructed patella ligament. Therefore, our institute follows a unique mobilisation protocol following proximal tibia and knee replacement which is depicted in the Table 2. In few cases quadriceps lag could be evident due to patella tendon overstretch/avulsion, this could be due to improper patella mobilisation or forceful knee bending. Fig. 3 a, b and c depicts the patella tendon overstretch.
Mega prosthetic replacement for proximal and total femoral resection:
Resection of proximal femur and prosthetic replacement may be done for proximal femur tumor or as a part of total femoral resection and reconstruction. Partial or complete loss of joint capsule and dynamic stabilisers of hip joint during tumor resection may leave the hip joint vulnerable to dislocation. This may get potentiated with certain combination movements, if these joint movements are allowed beyond a certain limit. This restriction largely depends upon the surgical approach. Postero-lateral approach being most common in the LSS of this site, hip rotations, especially internal rotation and, flexion more than 600 and adduction of the hip joint needs to be prevented up to 6 weeks [4,7]. These movement restrictions could be achieved using hip abduction pillow/brace and de-rotation splint. Before the patient gets discharged from the hospital, training them for bed transfer, supine to standing, standing to supine and sitting in a chair/commode becomes paramount important in the early phase of rehabilitation. Knee joint mobilisation must be started early either by the edge of bed with hip joint well supported or in side lying with pillows between legs. Any restriction of knee joint range would adversely affect the overall function since hip joint function of the ipsilateral leg has already been compromised. Early FWB ambulation could be started from post operative day one initially with walker, then with walking stick. Later most of them would be trained to walk without any walking aid. Total femoral resections may require more intense rehabilitation with additional emphasis on knee strengthening as discussed earlier (Table 2). Patients may life long need to use walking aids in view of the fact that large motor loss in these cases.
Mega prosthetic replacement for proximal humeral resections:
Proximal humerus and the shoulder girdle are the third common place for primary bone sarcomas [4]. Despite endoprosthetic replacements for functional shoulder girdle structures, such as reverse glenoid prosthesis, are available, lack of muscular structures post excision and damage to the axillary nerve often prevent their use. Frequently, the proximal end of the humerus is replaced with the endoprosthesis and suspended by the remaining muscles and soft tissue structures by suturing around the proximal end of the prosthesis. The objective of the procedure is to achieve a stable shoulder to facilitate good elbow and hand function.
To prevent the weight of the endoprosthesis and the limb acting on the newly formed pseudo joint, shoulder sling and elbow pouch are provided for 4 – 6 weeks. Early post operative rehabilitation consists of elbow and hand range of motion (ROM) and strengthening exercises within pain limit. Again these exercises must be performed in supine position only to avoid undue stress on the shoulder. After six weeks, shoulder joint limited ROM exercise in the form of pendular movements and vigorous strengthening of shoulder girdle, elbow and hand complex should be commenced. Additionally, postural correction must be included in the rehabilitation program.
Biological reconstructions
Wherever feasible, biological reconstructions are preferred over endoprosthetic implants in order to provide a stable and permanent solution for reconstruction of defects after tumor resection. However, rehabilitation following biological reconstructions needs careful considerations regarding weight bearing and joint mobilization. Utmost importance to surgical notes and communication with operative surgeon is of prime importance. Early joint mobilisation is the key to prevent joint stiffness and functional loss; nevertheless, often protective functional braces may be required to prevent damage. For example, curettage and bone grafting of the lower end of femur close to the joint demands hinge knee brace to avoid varus and valgus stress. Strengthening exercises also could be started early with functional knee brace (Fig. 4).
Patients are taught to walk non-weight bearing with brace generally from post operative day one with the help of axillary crutches up to 8 weeks. Once, osteosynthesis is confirmed through radiological evaluation, progressive weight bearing walking could be started. FWB walking and complete joint range and strength are expected to be achieved by the end of 3 months to 4 months.
conclusions
Although limb salvage surgery for primary malignant tumours have achieved commendable advancement in surgical techniques and endo-prosthetic design and manufacturing, without optimal and timely peri and post-operative physical rehabilitation, achieving the desired quality of life outcome may not be feasible. This paper has highlighted few important rehabilitation principles and we have summarised rehabilitation protocol for specific area. Most of the oncology resection and reconstruction vary from one individual to another even in one particular site and needs tailor made rehabilitation protocol; nevertheless, this summary will be a guide for necessary foundation to design individual rehabilitation program.
References
1. Chopra BK. Health related quality of life studies in Indian patients after limb salvage surgery: Need of the hour. Med J Armed Forces India. 2013 Jul;69(3):209–10.
2. Ottaviani G, Robert RS, Huh WW, Palla S, Jaffe N. Sociooccupational and physical outcomes more than 20 years after the diagnosis of osteosarcoma in children and adolescents. Cancer. 2013;119(20):3727–36.
3. Frieden RA, Ryniker D, Kenan S, Lewis MM. Assessment of patient function after limb-sparing surgery. Arch Phys Med Rehabil. 1993 Jan;74(1):38–43 6p.
4. Oren R, Zagury A, Katzir O, Kollender Y, Meller I. Principles of rehabilitation after limb-sparing surgery for cancer. In: Musculoskeletal Cancer Surgery [Internet]. Springer; 2001 [cited 2014 Sep 3]. p. 583–93. Available from: http://link.springer.com/chapter/10.1007/0-306-48407-2_36
5. Bekkering WP, Vliet Vlieland TPM, Fiocco M, Koopman HM, Schoones JW, Nelissen RGHH, et al. Quality of life, functional ability and physical activity after different surgical interventions for bone cancer of the leg: A systematic review. SurgOncol. 2012 Jun;21(2):e39–47.
6. Mei J, Zhu X-Z, Wang Z-Y, Cai X-S. Functional outcomes and quality of life in patients with osteosarcoma treated with amputation versus limb-salvage surgery: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014 Nov;134(11):1507–16.
7. Shehadeh A, Dahleh ME, Salem A, Sarhan Y, Sultan I, Henshaw RM, et al. Standardization of rehabilitation after limb salvage surgery for sarcomas improves patients’ outcome. HematolOncol Stem Cell Ther. 2013 Sep;6(3–4):105–11.
8. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010 Jul;42(7):1409–26.
9. Singh F, Newton RU, Galvão DA, Spry N, Baker MK. A systematic review of pre-surgical exercise intervention studies with cancer patients. SurgOncol. 2013 Jun;22(2):92–104.
10. Silver JK. Cancer Prehabilitation and its Role in Improving Health Outcomes and Reducing Health Care Costs. SeminOncolNurs. 2015 Feb;31(1):13–30.
11. Silver JK. Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. SeminOncolNurs. 2015 Feb;31(1):13–30.
| How to Cite this article: Paramanandam VS, Daptardar AA, Gulia A. Rehabilitation Following Limb-Salvage Surgery In Sarcoma. Journal of Bone and Soft Tissue Tumors May- Aug 2016;2(2):20-24. |

Like this:
Like Loading...






