Journal of Bone and Soft Tissue Tumors (JBST) Editorial Office
For any queries please contact the editorial office
Journal of Bone and Soft Tissue Tumors (JBST)
Ediotrial office, IORG House, A-203, Manthan Apts, Shreesh CHS, Hajuri Road, Thane [W]
Maharashtra, India. Pin 400604
Tel- 02225834545 (office time 10-5 pm, Mon-Fri)
Email: editor.jbst@gmail.com
Publisher: Indian Orthopaedic Research Group. Thane, India. 400604
Email: indian.ortho@gmail.com | Tel: 022-25834545
Important Information
Journal of Bone and Soft Tissue Tumors (JBST) License
![]()
Journal of Bone and Soft Tissue Tumors (JBST) by http://jbstjournal.com/ is licensed under https://creativecommons.org/licenses/by-nc-sa/4.0/
Based on a work at http://jbstjournal.com/.
Permissions beyond the scope of this license may be available at editor.jbst@gmail.com
Journal of Bone and Soft Tissue Tumors (JBST) is the official Journal of The Indian Musculo Skeletal Oncology Society


Guest Editorial – Asia Pacific Musculoskeletal Tumor Society Conference 2018
Vol 4 | Issue 2 | July-Dec 2018 | page: 2-3 | Ajay Puri.
Author: Ajay Puri [1].
[1] Orthopedic Oncology Services, Department of Surgical Oncology, Tata Memorial Hospital, Mumbai.
Address of Correspondence
Dr. Ajay Puri,
Department of Orthopaedic Oncology, Room No: 26, Tata Memorial Hospital, Borges Road, Parel, Mumbai – 400 012. India.
Email: docpuri@gmail.com
Asia Pacific Musculoskeletal Tumor Society Conference 2018
For advances in musculo skeletal oncology to occur we need a host of clinical specialties along with nursing, rehabilitation and support staff interacting on a continuous basis. Sarcomas are rare and each individual and institution can only benefit from having access to pooled information and experience. For sarcoma care to evolve, ideas to surface and multi institute or multi-disciplinary collaborations to develop in the fields of basic research, patient care, biomaterials and prosthesis, there is a need for a common platform where all of us involved in the treatment of sarcomas can interact. Our socio economic milieu in the Asia Pacific region is unique. We have a spectrum ranging from resource rich societies with extensive state supported health care to resource constrained nations where patients have inconsistent access to health care and funding for health care is limited. Solutions and protocols applicable to the Western world need not necessarily be the most suitable in our scenario. Our large numbers coupled by the sheer native ingenuity necessary to offer quality health care in a resource challenged population can help us develop solutions that can be adapted everywhere.
The Asia Pacific Musculoskeletal Tumor Society (APMSTS) was conceptualized during discussions at the 7th International Symposium of Limb Salvage Surgeons (ISOLS) meeting in 1993 and its first meeting was held in 1995 in Japan under the stewardship of Professor Yoshio Ogihara. Mirroring a similar initiative, the Indian Musculo Skeletal Oncology Society (IMSOS) was established in 2013 to “promote scientific, evidence based, comprehensive multidisciplinary management of bone and soft tissue sarcomas and encourage basic and clinical research”. This year Jaipur, India plays host to the 12th APMSTS meeting in October. This is a joint meeting co-hosted with IMSOS. The theme of the conference is “Education – Collaboration – Innovation”. This epitomizes our desire to share and disseminate knowledge, the spirit of collaboration necessary to find answers to common problems and the need to derive innovative solutions best suited to our socio economic milieu.
The conference logo depicts the feathers of the peacock in the Indian national colours adorning a group of people with interlinked arms who represent the collaboration and camaraderie between individuals and countries that form the Asia Pacific Musculoskeletal Society. The logo represents the beauty and spirituality of India while signifying the desire to showcase skills from the Asia Pacific region. The peacock, the national bird of India symbolizes many of the qualities that we as “healers” must inculcate. In Hinduism, the peacock is associated with Saraswati the goddess of wisdom and learning. In Buddhism, the peacock is a symbol of purity and the ‘eyes’ in the peacock’s tail represent a symbol of watchfulness. The figure of the peacock is painted in various Islamic religious buildings while in Christianity the peacock was known as the symbol of resurrection and renewal. Just as the peacock elegantly unfolds its vibrant colours during its ritual dance, so too this conference is an occasion for us to demonstrate the best from all participating delegates.
We have more than 300 delegates from 28 countries participating in this meeting. Deviating from the accepted norm of didactic lectures the meeting focusses more on interactive sessions that will promote healthy discussion. There are case based panel discussions and sessions where senior musculoskeletal oncologists share their diverse experiences so that all attendees can benefit from the collective experience of others in order to enable them to make better decisions when managing similar cases. While providing adequate sessions for free papers there are two “show piece” orations. The IMSOS oration “Lessons learned from the European-American Osteosarcoma Study EURAMOS1” will be delivered by Prof. Stefan Bielack while the inaugural APMSTS “Prof. Yoshio Ogihara Oration” will be delivered by Dr. Suresh Nathan. The conference is preceded by multidisciplinary workshops that encompass all specialties involved in sarcoma care, offering delegates an opportunity to interact with faculty in smaller groups.
This unique joint meeting also offers the ideal platform to unveil a brief monograph “IMSOS guidelines for musculoskeletal sarcomas”, IMSOS’s endeavour to standardise the management of musculoskeletal sarcomas in India.
All our efforts are ultimately focussed on improving health care for our patients. A meeting such as this would be incomplete unless we involved them too. The IMSOS sarcoma support initiative will simultaneously conduct a parallel session that helps patients share their experience and presents an occasion where we can felicitate support personnel who selflessly give their time to enable us to optimise overall patient care.
We are sure the ambience and hospitality of Jaipur combined with an exciting and informative scientific programme will make this joint APMSTS – IMSOS conference a memorable and enriching event for all delegates.
It is our pleasure and privilege to welcome all attendees to Jaipur to partake of the experience that is “Incredible India”!
Prof. Ajay Puri
President – Indian Musculo Skeletal Oncology Society
President – Asia Pacific Musculoskeletal Tumor Society
(Abstract Full Text HTML) (Download PDF)
Like this:
Denosumab Therapy Related Changes in Giant cell Tumor (Osteoclastoma) of Bone : A New Osteosarcoma Mimicker
Vol 4 | Issue 2 | July-Dec 2018 | Page 4-6 | Pradnya Manglekar, Sujit Joshi, Yogesh Panchwagh.
Authors: Pradnya Manglekar [1], Sujit Joshi [1], Yogesh Panchwagh [1].
[1] Dept. of Pathology Deenanath Mangeshkar Hospital and Research Centre, Pune
[2] Orthopaedic Oncology Clinic, Pune, India.
Address of Correspondence
Dr. Sujit Joshi,
Dept. of Pathology Deenanath Mangeshkar Hospital and Research Centre, Pune
Email: sujitjoshi30@gmail.com
Abstract
Objectives: Giant cell tumor (Osteoclastoma)of bone is locally aggressive osteolytic tumor. Denosumab–A monoclonal antibody against RANKL is recently being used to treat this tumor. We discuss histopathological changes occurring in giant cell tumor of bone after Denosumab treatment.
Method: A retrospective study of 12 cases from January 2014 to March 2018. All patients included were diagnosed as giant cell tumor (osteoclastoma) of bone on needle or open biopsy. Subsequently these patients received Denosumab therapy followed by surgical resection (extended curettage or wide excision). Histomorphological features after Denosumab therapy were evaluated in these specimens and compared with morphological features of prior biopsy samples.
Results: Needle or open biopsy samples studied prior to receiving Denosumab therapy showed typical morphological features of osteoclastoma i.e. presence of uniformly distributed osteoclastic cells and sheets of mononuclear stromal cells. No atypical mitoses or matrix production noted. Post Denosumab therapy resection specimens showed marked reduction in number of osteoclastic giant cells. There was predominance of mononuclear stromal cells along with abundant, irregular new bone (osteoid) formation with osteoid matrix deposition. Occasional mitotic activity was seen. Few foci of necrosis were noted.
Conclusion: Denosumab treatment causes significant giant cell depletion accompanied by abundant new bone formation separated by cellular stromal proliferation. These features bear very little resemblance to their pre-treatment counterparts and can mimic morphological features of osteosarcoma and other bone forming tumors. Hence, one should be aware of these changes so that a misdiagnosis of osteosarcoma can be avoided.
Keywords: Giant cell tumor (osteoclastoma), Denosumab, RANKL.
References
1. Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease.9th edition. Saunders Elsevier: Philadelphia; 2015. Chapter 26, Bones, Joints and Soft tissue tumors; p1179 – 1226.
2. Rosai J. Rosai and Ackerman’s Surgical Pathology. 10thedition. Elsevier Mosby: St.Louis, Missouri; 2011. Chapter 24, Bone and joints; p2013-2104.
3. Branstetter DG, Nelson SD, Manivel JC, Blay JY et al. Denosumab induces Tumor Reduction and Bone formation in Patients with Giant-Cell Tumor of Bone. Clin Cancer Res; 18(16); 4415-24.2012 AACR.
4. Singh AS, Chawla NS, Chawla SP. Giant-cell tumor of bone: treatment options and role of denosumab. Biologics: Targets and Therapy 2015;9, 69-74.
5. Huang L, Teng XY, Cheng YY, Lee KM, Kumta SM. Expression of preosteoblast markers and Cbfa-1 and Osterix gene transcripts instromal tumour cells of giant cell tumour of bone. Bone 2004 Mar;34 (3):p393–401.
6. James IE, Dodds RA, Olivera DL, Nuttall ME, Gowen M. Human osteoclastoma-derived stromal cells: correlation of the ability to form mineralized nodules in vitro with formation of bone in vivo. J Bone Miner Res 1996 Oct;11 (10):1453–60.
7. Wojcik J, Rosenberg AE, Bredella MA, et al. Denosumab-treated giant cell tumor of bone exhibits morphologic overlap with malignant giant cell tumor of bone. Am J SurgPathol 2016 Jan; 40(1): 72-80.
8. Rekhi B, Verma V, Gulia A, et al. Clinicopathological features of a series of 27 cases of post-denosumab treated giant cell tumors ofbones: a single institutional experience at a tertiary cancer referralcentre, India. PatholOncol Res 2017 Jan; 23 (1): 157-64.
9. Chang-Che Wu, Pin-Pen Hsieh. Denosumab-Treated Giant Cell Tumor of the Bone Mimicking Low-Grade Central Osteosarcoma.Journal of Pathology and Translational Medicine 2018; 52: 133-135.

(Abstract Full Text HTML) (Download PDF)
Like this:
JBST- Special APMSTS 2018 Issue
Vol 4 | Issue 2 | July-Dec 2018 | page:1 | Dr. Yogesh Panchwagh, Dr. Ashish Gulia, Dr. Ashok Shyam.
Author: Yogesh Panchwagh [1], Ashish Gulia [2], Ashok Shyam [1,3].
[1] Orthopaedic Oncology Clinic, Pune, India.
[2] Orthopedic Oncology Services, Department of Surgical Oncology, Tata Memorial Hospital, Mumbai.
[3] Indian Orthopaedic Research Group, Thane, India,
[4] Sancheti Institute for Orthopaedics &Rehabilitation, Pune, India
Address of Correspondence
Dr. Yogesh Panchwagh.
Orthopaedic Oncology Clinic, 101, Vasant plot 29, Bharat Kunj Society -2, Erandwana, Pune – 38, India.
Email: drpanchwagh@gmail.com
JBST- Special APMSTS 2018 Issue
It gives us immense pleasure in presenting the special IMSOS (Indian Musculoskeletal Oncology Society) issue of Journal of bone and soft tissue tumors on the occasion of the 12th Asia Pacific Musculoskeletal Tumor Society meeting, Jaipur, India. It’s only the second time, the first being in 2002, that APMSTS conference is being held in India. It is being co-hosted by the IMSOS, a relatively new body comprising of mostly the young but enthusiastic clinicians and paramedical staff involved in sarcoma care in India. Under the able presidentship of Dr Ajay Puri, a veteran in the field, the 12th APMSTS is all set to give the delegates a memorable experience. It’s but natural that Dr Ajay Puri, President – APMSTS 2018 and IMSOS has penned the guest editorial for this issue.
JBST (Journal of bone and soft tissue tumors) is proud to be associated with IMSOS as its official journal. All the members of IMSOS have contributed wholeheartedly to this issue echoing the theme of APMSTS 2018: “Education – Collaboration – Innovation”. The issue comprises of original articles on the data from institutes located across India on varied topics ranging from demographics of sarcoma in eastern India to osteosarcoma of facial bones from South India to recent topics like post denosumab changes in the histopathology of Giant cell tumors from western India. Even the case reports, which are rare clinical presentations of pathologies like osseous hydatid cyst, chordoma, clear cell chondrosarcoma, sacral inclusion cyst, hold a high educational value.
We also take this opportunity to invite all delegates to submit to Journal of Bone and Soft tissue tumors, which is one of the few journals that publish bone tumor research. We have a great editorial board and an online submission and review system. JBST has completed four years now and we are committed to make JBST the best resource for publishing bone tumor research in the world. This can be only achieved by co-operation and collaboration from all of you
We are happy to present this issue at the 12th APMSTS at Jaipur. Hope you will enjoy it as much as the vibrant, colourful and warm Indian hospitality.
Dr. Yogesh Panchwagh
Dr. Ashish Gulia
Dr. Ashok Shyam
(Abstract Full Text HTML) (Download PDF)
Like this:
Need for a dedicated Musculoskeletal Tumor Registry in India
Vol 3 | Issue 1 | May- Aug 2017 | page:1 | Dr. Yogesh Panchwagh & Dr. Ashok Shyam.
Author: Dr. Yogesh Panchwagh [1], Dr. Ashok Shyam [2,3].
[1]Orthopaedic Oncology Clinic, Pune, India.
[2] Indian Orthopaedic Research Group, Thane, India
[3] Sancheti Institute for Orthopaedics &Rehabilitation, Pune, India
Address of Correspondence
Dr. Yogesh Panchwagh.
Orthopaedic Oncology Clinic, 101, Vasanth plot 29, Bharat Kunj Society -2, Erandwana, Pune – 38, India.
Email: drpanchwagh@gmail.com
Need for a dedicated Musculoskeletal Tumor Registry in India
Registries are one of the most important resources for any disease. Around 200 cancer registries in the world that covering up mere 5% of worlds population was noted in 1999. However no parallel data exists currently but we are sure that number of cancer registries have increased over the period of time.India has an already existing National cancer registry program which was started in 1981 and is still running strong. However we belive that time has now come for a dedicated musculosketal registry in India. The technology has grown rapidly and now we have developed a simple mobile app which can collect most of the registry data in a very simple and user friendly way. We have been working on this concept sicne two years now and have taken inputs from many orthoonco surgeons. The mobile app is finally launched in the public domain and is available on google play store and app store. The app is named ‘MSK Registry’ and It can be downloaded for free by all interested in contributing cases. We would urge everyone to contribute and also write to us for any issues or queries
Regards.
Dr Yogesh Pachwagh
Dr Ashok Shyam
Yogesh Panchwagh & Ashok Shyam
Registry in India. Journal of Bone and Soft Tissue Tumors Sep-Oct 2017; 3(1):1.
(Abstract Full Text HTML) (Download PDF)
Like this:
Plantar Fibromatosis Masquerading as Liposarcoma : a Case Report and Review of Literature
Volume 2 | Issue 2 | May-Aug 2016 | Page 25-29 | Nishit Bhatnagar, Purushotham Lingaiah, Sumit Arora, Anil Dhal.
Authors: Nishit Bhatnagar [1], Purushotham Lingaiah [1], Sumit Arora [1], Anil Dhal [1].
[1]Department of Orthopaedic Surgery, Maulana Azad Medical College & associated Lok Nayak Hospital, New Delhi-110002
Address of Correspondence
Dr. Nishit Bhatnagar
7 Godavari Apartments, Alaknanda, New Delhi – 19
E-mail id: nishitbhatnagar@yahoo.co.in
Abstract
Plantar fibromatosis and well differentiated liposarcoma can have a similar clinical presentation of a slow growing superficial well defined mass on the plantar aspect of foot. We present a patient of plantar fibromatosis mimicking a lipomatous tumour of the foot. Marginal resection of the remaining tumour was performed. At two years postoperatively, there has been no recurrence of the tumour.
Keywords: liposarcoma, foot, plantar fibromatosis
Introduction
Plantar fibromatosis is a benign lesion involving the plantar aponeurosis. Ledderhose [1] in 1897 reported and described approximately 50 cases of contractures of the plantar fascia, leading to the entity being termed as Ledderhose’s disease. Its similarity to Dupuytrens’s disease of the hand has also led to the term “Dupuytren’s disease of the plantar fascia” [2]. It is commonly known to occur in the 3rd to 5th decade of life, has a male preponderance and is bilateral in 20 to 50% of cases [3,4,5]. It has an unknown etiology and is characterized by neoplastic proliferation of immature fibroblasts with spindle-shaped myofibroblasts within the plantar fascia [6]. They can be locally aggressive, demonstrate local recurrence but do not metastasize. Lipogenic tumours represent the most common soft tissue tumours [7]. Atypical and malignant lipomatous neoplasms are the most common variety of adult soft tissue sarcomas, accounting for nearly 20% of all sarcomas [8]. Lipomatous tumours are most frequently found in the extremities, retroperitoneum, groin and abdominal wall [9]. Lipomatous tumours can range from benign lipomas to highly malignant dedifferentiated liposarcomas [10]. Nomenclature and classification of lipomatous tumours has undergone major changes over time. Based on cytogenetic and molecular genetic studies, liposarcomas were classified by the World Health Organization Committee for classification of soft tissue tumours into five subtypes, atypical/well-differentiated liposarcoma, dedifferentiated liposarcoma, myxoid liposarcoma (including high grade round cell liposarcoma), pleomorphic liposarcoma and a rare mixed-type liposarcoma [8]. Some workers have proposed that the term ‘atypical lipomatous tumour’ should be used for tumours arising from extremities and chest wall, whereas ‘well differentiated liposarcoma’ should be used for describing tumours arising in the retroperitoneum and abdominal cavity [11,12,13]. Diagnosis of well differentiated liposarcomas of the extremities can be delayed due to relatively benign symptomatology and a low index of suspicion. Plantar fibromatosis and well differentiated liposarcoma can have a similar clinical presentation of a slow growing superficial well defined mass on the plantar aspect of foot. Here we present a patient of plantar fibromatosis mimicking a lipomatous tumour of the foot.
Case presentation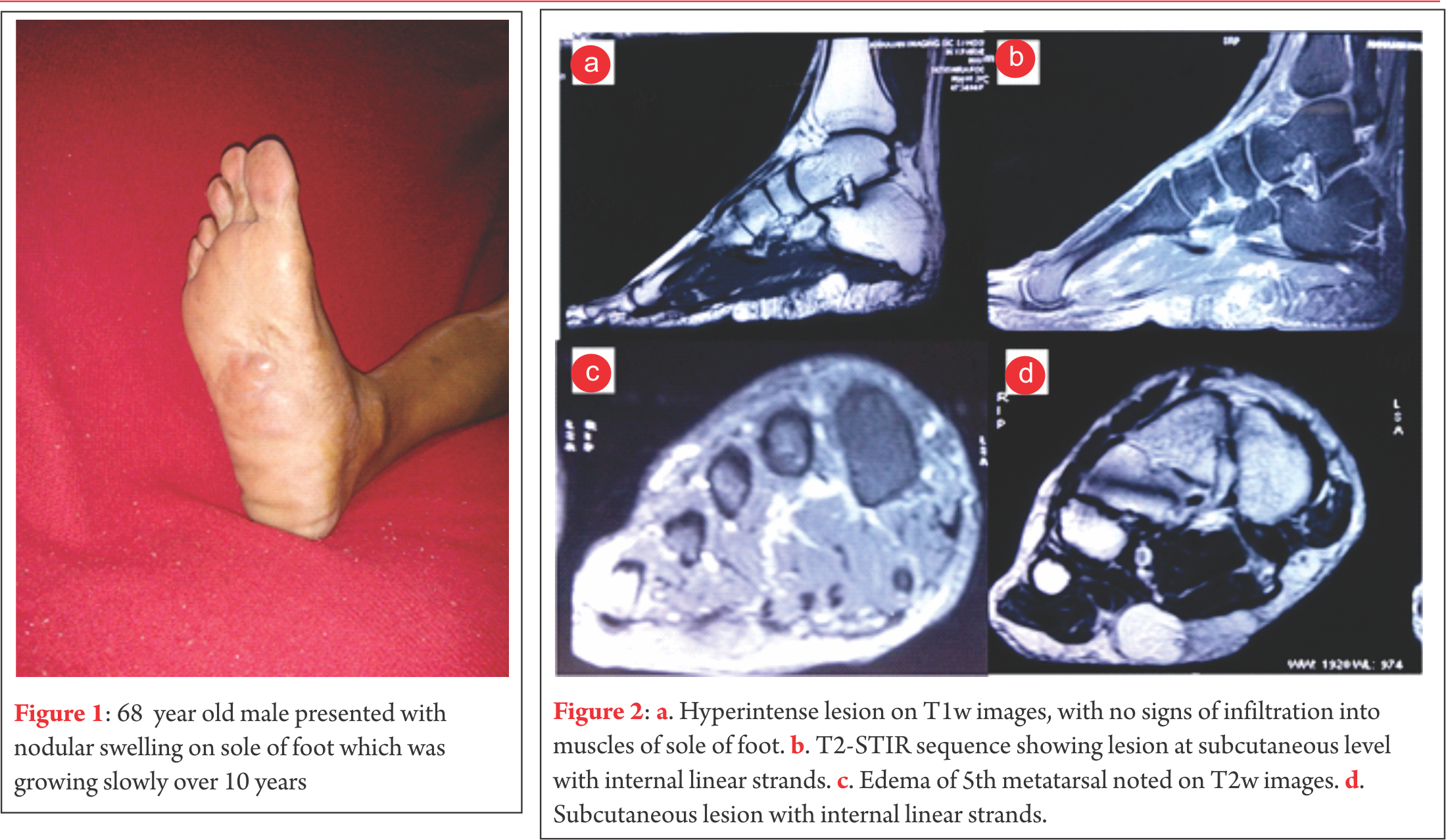 A diagnosis of plantar fibromatosis was made, keeping lipoma as a differential diagnosis. Plain radiographs showed no bony pathology. An unsuccessful trial of oral analgesics and customized insole was given. Prior to operative intervention for the mass on the plantar aspect of foot an excisional biopsy of the mass on the lateral border of the foot was performed. The biopsy revealed nodular non-encapsulated fibrofatty tissue on gross examination. Histopathological examination revealed nodular proliferation of adipocytes and myofibroblasts separated by collagenous bands. The nodules of fatty tissue showed a mixture of mature and immature fat cells with multivacuolation and nuclear indentation. Nuclear pleomorphism, hyperchromasia and mitosis were observed leading to a diagnosis of well differentiated liposarcoma. MRI revealed a 6.5cm x 6.6cm x 2cm well circumscribed, multilobulated lesion along the plantar aspect of the mid foot at the subcutaneous level that appeared to be indistinguishable from surrounding subcutaneous fat with internal linear strands. It was seen to insinuate between medial and lateral heads of plantar aponeurosis, however it did not show infiltration into the muscles of sole of the foot. Overlying skin of sole of foot appeared uninvolved. The lesion was hyperintense on T1w images, however suppresses completely on T2-STIR sequences. Few linear strands are noted tha are isointense in T1w images and showed enhancement in post contrast images (Fig. 2). Except for subtle focal edema in the base of the fifth metatarsal there was no bony involvement. Marginal resection of the remaining tumour was performed through an S-shaped incision on the non-weight bearing area of the sole. Tumour tissue was found to be lying between the dermis and the plantar aponeurosis. It was observed to be comprised of friable adipose tissue with a few intervening fibrotic septae. Tumor was non-encapsulated, however it could be easily separated from rest of the neighbouring structures. The adjacent aponeurosis and dermis appeared to be free of any gross tumour infiltration. The medial plantar neurovascular bundle also appeared free from any tumour infiltration. Histology revealed nodules of mature and immature adipose tissue separated by fibrovascular septae with areas of increased cellularity, pleomorphism, hyperchromasia and tumour giant cells (Fig. 3).
A diagnosis of plantar fibromatosis was made, keeping lipoma as a differential diagnosis. Plain radiographs showed no bony pathology. An unsuccessful trial of oral analgesics and customized insole was given. Prior to operative intervention for the mass on the plantar aspect of foot an excisional biopsy of the mass on the lateral border of the foot was performed. The biopsy revealed nodular non-encapsulated fibrofatty tissue on gross examination. Histopathological examination revealed nodular proliferation of adipocytes and myofibroblasts separated by collagenous bands. The nodules of fatty tissue showed a mixture of mature and immature fat cells with multivacuolation and nuclear indentation. Nuclear pleomorphism, hyperchromasia and mitosis were observed leading to a diagnosis of well differentiated liposarcoma. MRI revealed a 6.5cm x 6.6cm x 2cm well circumscribed, multilobulated lesion along the plantar aspect of the mid foot at the subcutaneous level that appeared to be indistinguishable from surrounding subcutaneous fat with internal linear strands. It was seen to insinuate between medial and lateral heads of plantar aponeurosis, however it did not show infiltration into the muscles of sole of the foot. Overlying skin of sole of foot appeared uninvolved. The lesion was hyperintense on T1w images, however suppresses completely on T2-STIR sequences. Few linear strands are noted tha are isointense in T1w images and showed enhancement in post contrast images (Fig. 2). Except for subtle focal edema in the base of the fifth metatarsal there was no bony involvement. Marginal resection of the remaining tumour was performed through an S-shaped incision on the non-weight bearing area of the sole. Tumour tissue was found to be lying between the dermis and the plantar aponeurosis. It was observed to be comprised of friable adipose tissue with a few intervening fibrotic septae. Tumor was non-encapsulated, however it could be easily separated from rest of the neighbouring structures. The adjacent aponeurosis and dermis appeared to be free of any gross tumour infiltration. The medial plantar neurovascular bundle also appeared free from any tumour infiltration. Histology revealed nodules of mature and immature adipose tissue separated by fibrovascular septae with areas of increased cellularity, pleomorphism, hyperchromasia and tumour giant cells (Fig. 3). 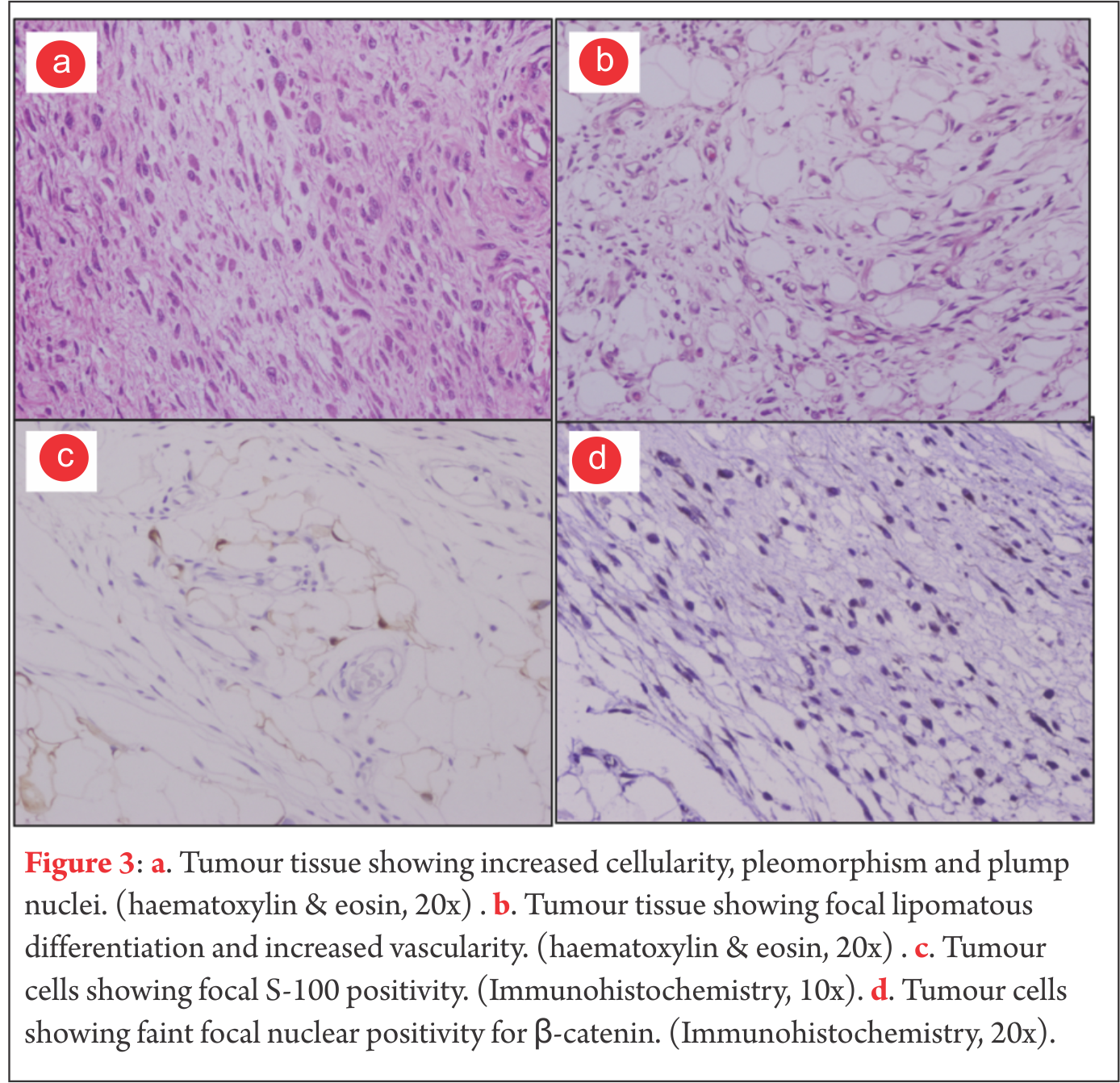 Mitotic figures were rare. Occasional cell with lipoblast like morphology was seen. Focal S-100 positivity was seen. Fatty tissue adherent to the skin also showed lesion cells in them. Due to proximity of tumour to the skin of the sole, thin skin flaps had to be created. Postoperatively marginal skin necrosis was observed and wound healing was delayed. Patient did not receive any postoperative chemotherapy or radiotherapy. At two years postoperatively, there has been no recurrence of the tumour and patient is able to ambulate without any discomfort. Patients consent was solicited before publishing the report
Mitotic figures were rare. Occasional cell with lipoblast like morphology was seen. Focal S-100 positivity was seen. Fatty tissue adherent to the skin also showed lesion cells in them. Due to proximity of tumour to the skin of the sole, thin skin flaps had to be created. Postoperatively marginal skin necrosis was observed and wound healing was delayed. Patient did not receive any postoperative chemotherapy or radiotherapy. At two years postoperatively, there has been no recurrence of the tumour and patient is able to ambulate without any discomfort. Patients consent was solicited before publishing the report
A 68-years-old male of Indian subcontinent was referred to our department with a ten year history of slow growing nodular masses on the non-weight bearing part of sole and along the lateral border of the right foot. The mass on the sole of the foot had become painful over the last one year. The masses were superficial, well defined, mildly tender and soft to firm in consistency (Fig. 1).
Discussion
Liposarcomas of the extremities are the second most commonly encountered soft tissue sarcoma after fibrous / fibrohistiocytic malignancies [14]. They occur almost exclusively in the age group between 40 to 60 years of age [14,15]. Liposarcomas are commonly encountered in the lower extremity, usually in the thigh, but rarely in the foot. Enzinger et al analyzed 1067 cases of liposarcoma, but none of them were in the foot [16]. There have been very few cases of liposarcomas of the foot reported in literature [17,18,19,20]. Preoperative diagnosis is infrequent. Hence, increasing the understanding of this tumour is important. WHO has categorized liposarcoma into five subtypes, out of which the well-differentiated variety is the most common, accounting for approximately 40% to 50% of all liposarcomas [21,14,22]. According to Evans et al a ‘well-differentiated liposarcoma’ and ‘atypical lipoma’ are identical in biological, behaviour, histological and karyotypic characteristics [23]. The term well differentiated liposarcoma is better used for lipomatous lesion in regions where wide resection is not possible (mediastinum and retroperitoneum), whereas the tumour is considered to be an atypical lipoma in other locations. Clinically, liposarcomas usually present as a painless soft tissue mass. Only around 10-15% liposarcomas present as a painful soft tissue mass [14]. These can be easily confused with fibromatosis which presents as slow growing single or multiple painful nodular thickenings [24]. A lipoma and a well-differentiated liposarcoma are also quite similar in clinical presentation. MR imaging is an essential tool for detection of liposarcoma as well as for studying its locoregional extension and relations (bone, soft-tissues and neurovascular involvement). MRI can help in differentiating between lipoma, liposarcoma and fibromatosis. Well-differentiated liposarcomas typically demonstrate a largely lipomatous mass, hyperintense on TW1 images, representing over 75% of the lesion in a nodular arrangement separated by thick non-lipomatous septae (>2mm but not exceeding 2cm). The non-lipomatous component shows variable enhancement on fat saturated T1w contrast enhanced images [25,26]. Lipomas also demonstrate abundant adipose tissue homogenously hyperintense on T1w images, similar to well-differentiated liposarcomas. However, lipomas have thin septae (<2mm) and contrast enhancement of lower signal intensity [21]. Fibromatosis on MRI shows nearly the same low-signal intensity as adjacent muscle on T1w and T2w images. Majority of fibromatosis show marked enhancement on gadolinium administration [27,28]. MRI must be performed prior to biopsy or any therapeutic management of a suspected liposarcoma. A well-differentiated liposarcoma appears grossly like a well-circumscribed multi-lobulated mass. Some sections of the tumour reveal mature adipose tissue in abundance that appears identical to a lipoma. However, a well-differentiated liposarcoma can be histologically identified by a typical scattering of lipoblasts with irregularly shaped hyperchromatic nuclei, along with thick fibrovascular septae [16]. Immunohistochemical analysis helps to distinguish a lipoma from a well-differentiated liposarcoma. MDM2 and CDK4 markers are expressed by a well-differentiated liposarcoma [29]. Fibromatosis can be identified based on its characteristic nodular cellular proliferation of plump, spindle shaped cells with intervening collagen with infrequent mitotic figures [30]. Well-differentiated liposarcomas are not known to have malignant potential but local recurrence risk is high [31]. For such tumours of the extremity, the local recurrence rate can be as high as 43% [32]. Such tumours are also reported to have undergone dedifferentiation into a more aggressive form with higher risk of local recurrence and metastasis [9].Prognosis and management of these tumours is related to their anatomical location. Most authors suggest that subcutaneously located tumours can be treated by wide resection, with minimal chances of local recurrence. Radiotherapy is not recommended unless there is gross residual tumour tissue. Chances of recurrence are much higher for tumours in deeper locations. Local recurrence can be treated by re-excision and radiotherapy [32,33,34]. Asymptomatic or mildly symptomatic lipomas of the extremity can be managed conservatively. Troublesome lipomas can be treated by simple excision. The management of fibromatosis can range from conservative management, intralesional steroid injections, collagenase injections, radiotherapy to surgical excision [35,36,37,38,39]. Due to such different lines of management of these pathologies, it is imperative to establish the correct diagnosis before proceeding to treatment. In this case, clinical presentation suggested a diagnosis of plantar fibromatosis, imaging studies suggested the possibility of a lipomatous tumour and histopathological evidence rasied the suspicion of a well differentiated liposarcoma. However, the final diagnosis of plantar fibromatosis was established after correlating the clinical features with a definitve histopathological evaluation following excision of the mass.
conclusions
Although plantar fibromatosis is a commonly encountered disease, it can be mimicked by rare pathologies like lipoma and liposarcoma. Hence, a high index of suspicion is required for their early diagnosis and proper surgical management. Management decisions should be taken after careful correlation between clinical, radiological and histopathological features.
References
1. Ledderhose H. Zur Pathologie der Aponeurose des Fusses und der Hand. Langenbecks Arch Klin Chir. 1897;55:694-712.
2. Cavolo DJ, Sherwood GF. Dupuytrens’s disease of the plantar fascia. J Foot Surg. 1982.21(1):12-15.
3. Weiss SW, Goldblum JR, Enzinger FM. Fibromatoses. In: Weiss SW, Goldblum JR editors. Enzinger and Weiss’ Soft Tissue Tumors. Philadelphia, Pa, USA: Mosby Elsevier;2008. p. 227–228.
4. Lee TH, Wapner KL, Hecht PJ. Plantar fibromatosis. J Bone Joint Surg Am. 1993;75(7):1080–1084.
5. Aviles E, Arlen M, Miller T. Plantar fibromatosis. Surgery. 1971;69(1):117–120.
6. Zgonis T, Jolly GP, Polyzois V, Kanuck DM, Stamatis ED. Plantar fibromatosis. Clin Podiatr Med Surg. 2005;22(1):11-18.
7. Mentzel T, Fletcher CD: Lipomatous tumours of soft tissues: An update. Virchow’s Arch. 1995; 427(4): 353-363.
8. Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon:IARC Press; 2002. p. 9-18.
9. Weiss SW, Rao VK. Welldifferentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites. A follow-up study of 92 cases with analysis of the incidence of “Dedifferentiation”. Am J Surg Pathol 1992;16(11):1051-1058.
10. Dei Tos AP. Liposarcoma: New entities and evolving concepts. Ann Diagn Pathol. 2000;4(4):252-266.
11. Fletcher CD. Soft tissue tumours. In: Fletcher CD, editor. Diagnostic histopathology of tumours. 2nd edition. London:Harcourt Publishers Limited; 2000:1473–1540.
12. Kindblom LG, Angervall L, Fassina AS. Atypical lipoma. Acta Pathol Microbiol Immunol Scand [A]. 1982;90(1):27–36.
13. Evans HL, Soule EH, Winkelmann RK. Atypical lipoma, atypical intramuscular lipoma, and well differentiated retroperitoneal liposarcoma: a reappraisal of 30 cases formerly classified as well differentiated liposarcoma. Cancer. 1979;43(2):574–584.
14. Peterson JJ, Kransdorf MJ, Bancroft LW, O’Connor MI. Malignant fatty tumours: classification, clinical course, imaging appearance and treatment. Skeletal Radiol. 2003;32(9):493-503.
15. Munk PL, Lee MJ, Janzen DL, Connell DG, Logan PM, Poon PY, Bainbridge TC. Lipoma and liposarcoma: evaluation using CT and MR imaging. AJR Am J Roentgenol. 1997;169(2):589-594.
16. Enzinger FM, Weiss SW. Liposarcoma. In: Weiss SW, Goldblum JR, Folpe AL, editors. Soft Tissue Tumors. 3rd edition. St. Louis, Mosby, USA: Mosby Elsevier;1995:686-697.
17. Sugar S, Murphy BM. Liposarcoma of the foot; case report. J Mich State Med Soc. 1955;54(4):468–469.
18. Kelly PC, Shramowiat M. Liposarcoma of the foot: a case report. J Foot Surg. 1978;17(1):27–31.
19. Rajasekhar C, Paul AS, Bale RS. Spindle-cell liposarcoma: a rare variant of liposarcoma arising in the foot. Clin Oncol (R Coll Radiol). 2005;17(2):128–129.
20. Matsuo T, Sugita T, Shimose S, Kubo T, Yasunaga Y, Ochi M. Liposarcoma arising in the foot: a case report. Case Rep Med. 2009;2009:630203.
21. Kansdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumours: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
22. Murphey MD, Arcara LK, Fanburg-Smith J. Imaging of musculosketal liposarcoma with radiologic-pathologic correlation. Radiographics 2005;25(5):13711395.
23. Evans H. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3(6):507-523.
24. Chokshi FH, Jose J, Clifford PD. Plantar fibromatosis. Am J Orthop. 2009;38(9):475-476.
25. Matsumoto K, Takada M, Okabe H, Ishizawa M. Foci of signal intensities different from fat in well-differentiated liposarcoma and lipoma: correlation between MR and histological findings. J Clin Imaging. 2000;24(1):38-43.
26. Galant J, Martí-Bonmatí L, Sáez F, Soler R, Alcalá-Santaella R, Navarro M. The value of fat-suppressed T2 or STIR sequences in distinguishing lipoma from well-differentiated liposarcoma. Eur Radiol. 2003;13(2):337-343.
27. Walker EA, Petscavage JM, Brian PL, Logle CI, Montini KM, Murphey MD. Imaging features of superficial and deep fibromatoses in the adult population. Sarcoma [Internet]. 2012;2012. Epub 2012 Jun 28, Article ID 215810, 17 pages. Available from Hindawi at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3395298/pdf/SRCM2012-215810.pdf
28. Morrison WB, Schweitzer ME, Wapner KL, Lackman RD. Plantar fibromatosis: a benign aggressive neoplasm with a characteristic appearance on MR images.Radiology. 1994;193(3):841-845.
29. Shimada S, Ishizawa T, Ishizawa K, Matsumura T, Hasegawa T, Hirose T. The value of MDM2 and CDK4 amplification levels using real time polymerase chain reaction for the differential diagnosis of liposarcomas and their histologic mimickers. Hum Pathol. 2006;37(9):1123-1129.
30. Fetsch JF, Laskin WB, Miettinen M. Palmar-plantar fibromatosis in children and preadolescents: a clinicopathologic study of 56 cases with newly recognized demographics and extended follow-up information. Am J Surg Pathol. 2005;29(8):1095-1105.
31. Forus A, Larramendy ML, Meza-Zepeda LA, Bjerkehagen B, Godager LH, Dahlberg AB, Saeter G, Knuutila S, Myklebost O. Dedifferentiation of a welldifferentiated liposarcoma to a highly malignant metastatic osteosarcoma:amplification of 12q14 at all stages and gain of 1q22-q24 associated with metastases. Cancer Genet Cytogenet. 2001;125(2):100-111.
32. Kindblom LG, Angervall L, Svendsen P. Liposarcoma a clinicopathologic, radiographic and prognostic study. Acta Pathol Microbiol Scand Suppl. 1975;(253):1-71.
33. Kang J, Botros M, Goldberg S, Giraud C, Nielsen GP, Chen YL, Raskin K, Schwab J, Yoon SS, Hornicek FJ, Delaney TF. The Use of Radiation Therapy in the Management of Selected Patients with Atypical Lipomas. Sarcoma [Internet]. 2013;2013. Epub on 2013 Jan 15, Article ID 485483, 5 pages. Available from Hindawi at: http://www.hindawi.com/journals/sarcoma/2013/485483/
34. Sommerville SM, Patton JT, Luscombe JC, Mangham DC, Grimer RJ. Clinical outcomes of deep atypical lipomas (well-differentiated lipoma-like liposarcomas) of the extremities. ANZ Journal of Surgery. 2005;75(9):803–806.
35. Sammarco GJ, Mangone PG. Classification and treatment of plantar fibromatosis. Foot Ankle Int 2000;21(7):563-569.
36. Hammoudeh ZS. Collagenase Clostridium histolyticum injection for plantar fibromatosis (Ledderhose disease). Plast Reconstr Surg. 2014;134(3):497e-498e.
37. Grenfell S, Borg M. Radiotherapy in fascial fibromatosis: a case series, literature review and considerations for treatment of early-stage disease. J Med Imaging Radiat Oncol. 2014;58(5):641-647.
38. McNally EG, Shetty S. Plantar fascia: imaging diagnosis and guided treatment. Semin Musculoskelet Radiol. 2010;14(3):334-343.
39. Aluisio FV, Mair SD, Hall RL. Plantar fibromatosis: treatment of primary and recurrent lesions and factors associated with recurrence. Foot Ankle Int. 1996;17(11):672-678.
(Abstract Full Text HTML) (Download PDF)
Like this:
Rehabilitation following Limb-Salvage Surgery in Sarcoma
Volume 2 | Issue 2 | May-Aug 2016 | Page 20-24 | Vincent S Paramanandam, Anuradha A Daptardar, Ashish Gulia
Authors: Vincent S Paramanandam [1], Anuradha A Daptardar [1], Ashish Gulia [2].
1Physiotherapy Department, Tata Memorial Hospital, Mumbai
2Orthopedic Oncology Services, Department of Surgical Oncology, Tata Memorial Hospital, Mumbai.
Address of Correspondence
Dr. Paramanandam V
Technichal Officer C, Physiotherapy Department, Tata Memorial Hospital, Mumbai
Email: vinsu24@gmail.com
Abstract
Introduction: Limb salvage after tumor resection has become a norm in today’s era. There are number of biological and non biological reconstruction options available for the reconstruction of these bone defects. The success story of these surgical procedures is mainly based on their excellent functional outcome. Post surgical rehabilitation plays an important role in achieving optimal functional outcome and good quality of life. The rehabilitation protocol following limb salvage surgery is complex and it differs with type of reconstruction procedure. Present articles discusses in detail the various rehabilitation protocols required to achieve above goals.
Keywords: Limb salvage surgery, rehabilitation, sarcoma
Introduction
Until 1970, amputation was the primary surgical treatment offered to bone and soft tissue sarcomas. However, from that time the treatment options have evolved dramatically and now approximately 90% of these cases undergo Limb Salvage Surgery (LSS)[1]. LSS has become the main line of treatment option for bone and soft tissue sarcomas along with adjuvant and/or neo adjuvant treatment modalities (Chemotherapy/ radiotherapy). The overall survival rate has been estimated as 55%-65%, based on the age of diagnosis, and it is considered to be comparable to that of amputation.
LSS is considered to be less invasive, provides better function and quality of life than amputation [2]. Moreover, it has been proposed that patients’ acceptability of LSS is high in view of the fact that it restores the body image better than amputation[3]. Nevertheless, LSS, unlike amputation, is associated with more peri-operative complications, prolonged hospital stay and requires repeated surgeries due to various reasons such as infection and prosthetic failure. LSS demands high surgical skills, whereas, amputation is a simple surgical procedure. Additionally, recent progress in prosthetic limbs, for example microprocessor based joints and endo-skeletal prosthetic reconstructions, have improved the functional outcome and cosmetic outlook following amputation [4]. A systematic review conducted by Bekkering et al [5] reported that the quality of life outcome from current available evidence is inconclusive in supporting LSS or amputation. Another recent systematic review and meta-analysis concluded that both surgical procedures provides similar functional recovery and quality of life [6].Despite the fact that early physical rehabilitation is the key to achieve good functional outcome and quality of life after LSS, rehabilitation techniques following LSS is largely neither tested nor documented in detail [7]. Lack of adequate early rehabilitation measures following LSS could be one of the rationales for conflicting interests reported by various studies examining the quality of life in LSS vs amputation.Hence, we have attempted to summarise basic principles and site specific considerations one must utilise to develop individual case specific rehabilitation protocol.
Common rehabilitation principles in LSS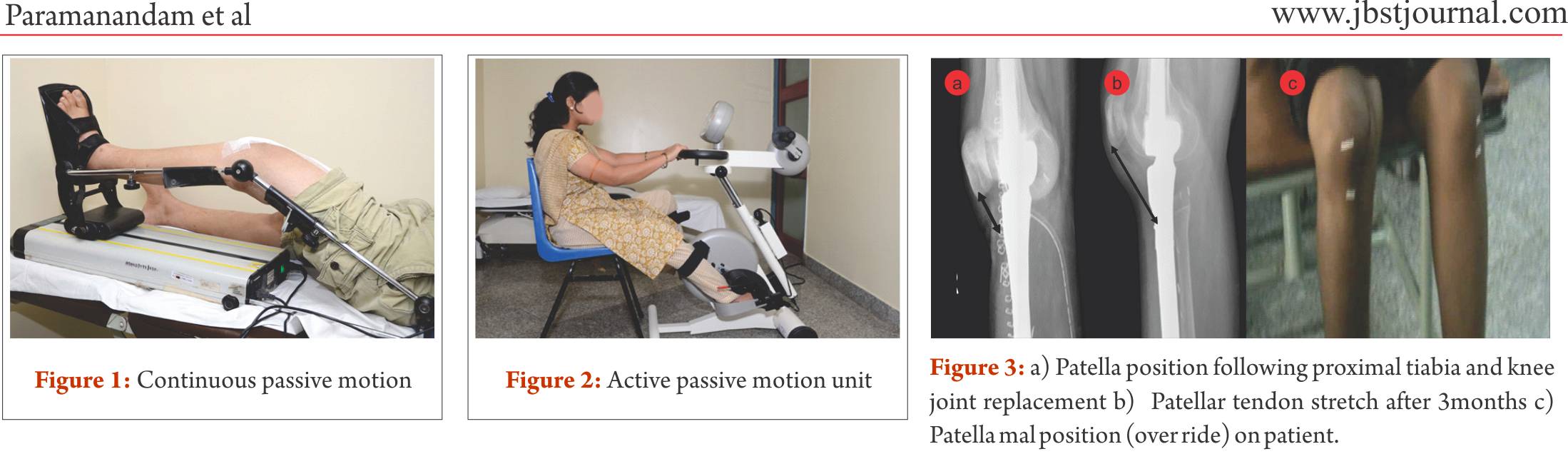
In a recent paper, Shehadeh et al. [7] attempted to standardize the rehabilitation protocol for LSS following high grade bone and soft tissue sarcomas. They reported that following a standardized rehabilitation protocol produced improved functional outcome in group of 59 patients with LSS. Their conclusion, however, is based on small observational study with heterogeneous population who received different type of LSS for different anatomical sites. Following set protocol in LSS, unlike general orthopaedic procedure, will be counterproductive. In general orthopaedic procedures, more or less,
specific anatomical structures are involved with minimal damage to the bone, joint and soft tissue structures. In contrast, in LSS following sarcomas, these structures are extensively resected and may not be identical between two individuals undergoing similar procedures for a particular site. For example, resection length for distal end of femur osteogenic sarcoma may depend on the extent of disease in two individuals [4]. Following are common rehabilitation prospectives that need to be considered to formulate comprehensive rehabilitation protocol for LSS.
Bone and joint reconstructions: Stability and mobility following LSS largely depends on the bone and joint structure loss and the type of reconstruction. For example, megaprosthetic distal femur replacement with cementing will allow the patient to be ambulated full weight bearing (FWB), whereas, if it is a bone graft , like in most of biological reconstructions, weight bearing needs to be delayed till the osteosynthesis is confirmed by radiographical evaluation.
Neuromuscular loss: Oncology resection demands large resections, which will also include a part of uninvolved soft tissue cover as surgical margin. Large resection may require additional rotational or free flaps for soft tissue coverage. In addition, nearby neuro-vascular bundle may need to be excised or repaired, hence, complete evaluation of neuro-motor loss would be necessary to plan the dynamic strength training and external support requirements.
Skin involvement: Donor sites of free flaps often receive split thickness skin graft which may hinder the early mobilisation of nearby joint. Moreover, scar development following open biopsy and LSS may need special attention from the rehabilitation team to prevent any future functional loss.
External supports: Temporary or permanent external support in the form of static or dynamic splinting may be required to provide support to the limb. To exemplify, prophylactic use of abduction brace along with derotation boot to prevent hip dislocation following proximal femur replacement and dynamic cock-up splint for radial nerve palsy needs to be the integral part of rehabilitation service.
Oncology treatments: Deranged blood count often hinders with the intensity of rehabilitation; hence, it prolongs the overall rehabilitation(8). Radiation induced fibrosis could cause severe restrictions in the joint range of motion. Thus, rehabilitation professionals must plan around the chemotherapy cycles and add prophylactic measures to prevent any impending radiation induced joint and soft tissue dysfunction.
Multidisciplinary approach: Limb salvage surgery is complex and demands close concordance in treatment specific outcomes between various health professional working in the rehabilitation team. This team may comprise of surgeons, medical oncologists, radiation therapists, nurses, physiotherapists, occupational therapists, prosthetics orthotics and medical social workers. Prehabilitation, rehabilitation even before starting the primary cancer therapy and surgery, such as crutch muscles strengthening, would be greatly beneficial in post-treatment functional outcome. Although in the field of LSS evidence of prehabilitation is lacking, there are considerable evidence to show beneficial effects in overall rehabilitation following cancer therapies [9–11].
Rehabilitation prescriptions and follow-up: Rehabilitation protocol for LSS must be tailor made considering the general principles and site specific modification, hence specific and also progressive. However, some negative effects of adjuvant therapies, such as the deranged blood counts and infection, may alter the course of rehabilitation process. Thus, frequent follow-up and close monitoring may be required during adjuvant therapy till they are functionally independent.
Rehabilitation consideration for specific sites
Site specific rehabilitation principles following LSS have been presented below for few common sites.
Mega prosthetic replacement for distal femoral resection:
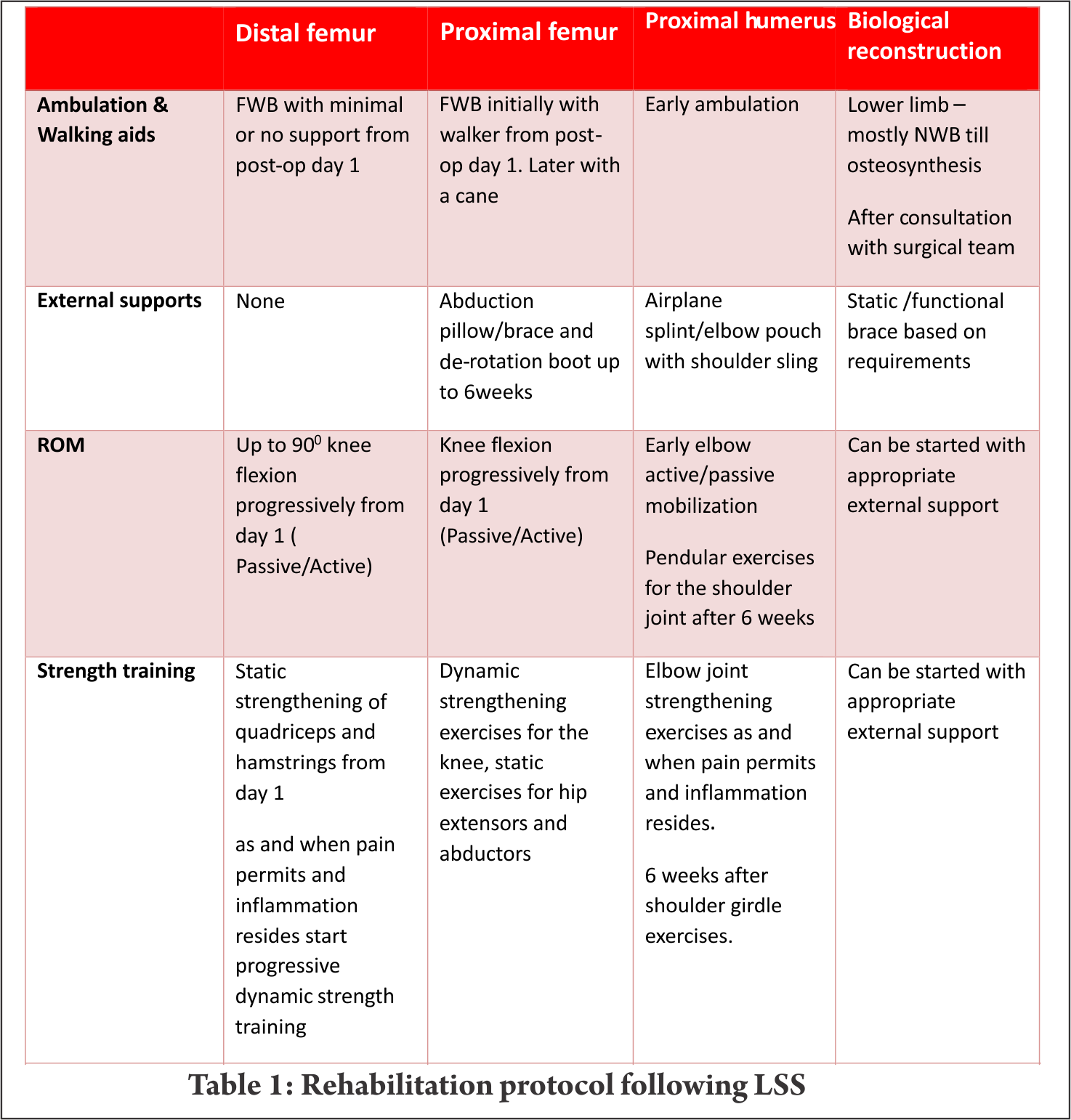
Distal femur is the commonest site for primary high grade sarcoma and giant cell tumors. Overall strengthening other than the affected site, in all possibility, should begin preoperatively. Limb elevation and ankle toe movement should be encouraged from post operative day one to prevent deep vein thrombosis. Cemented and semiconstrained (allows rotations and flexion/extension) knee joint endoprosthetic replacement permits early joint mobilization and FWB walking. Unlike other centres [4,7], in our centre knee joint mobilisation starts from day one with the help of continuous passive motion units (Fig.1) and active assisted methods within the pain tolerance level unless tight suturing. Close communication between the surgical team and the rehabilitation team helps in personalising the rehabilitation protocol as per the patients’ requirements. With adequate pain relief through appropriate medical management, active exercises could be started from day one to three. Full weight bearing walking could be started from day one initially with walker and later without any support if patient could effectively extend the knee “locking the knee”. Prior to ambulation, one leg standing and spot marching must be encouraged with appropriate support. After acute inflammation subsides slow progressive muscle strengthening exercises must be encouraged with the goal of achieving 900knee flexion, complete knee extension and muscle strength equivalent to the contra lateral lower limb by end of three months. Active passive motion devices, such as the one shown in Fig. 2, may help in joint mobilisation and strengthening. Summary of rehabilitation protocol is tabulated in Table 1.
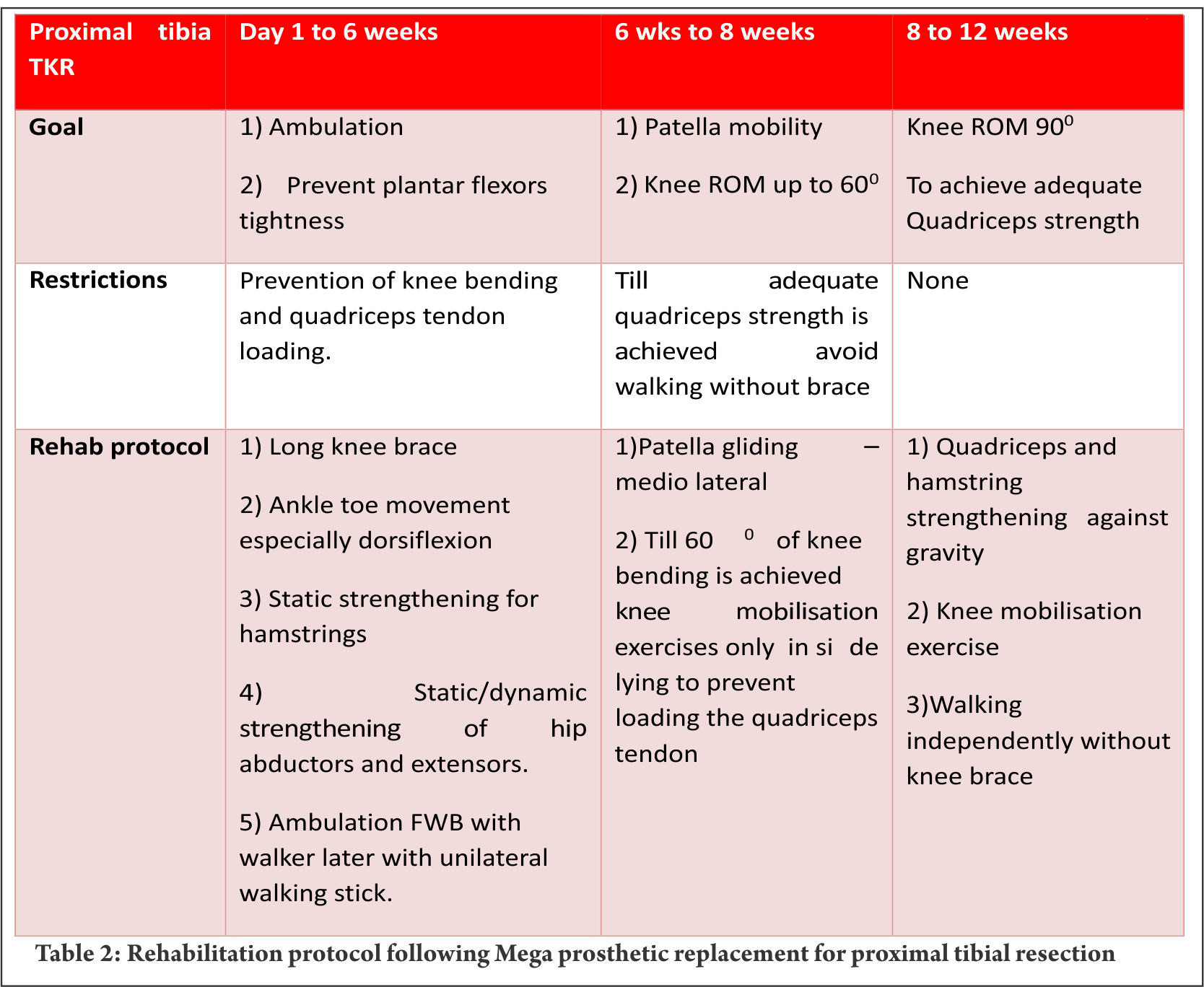
Mega prosthetic replacement for proximal tibial resection:
Proximal tibia and knee joint is the second most common site for primary high grade sarcoma and giant cell tumors. It is indeed a challenging location for rehabilitation in view of the fact that the extensor mechanism have to be reconstructed in these cases [4]. In most of the cases a gastrocnemius flap is done to provide a dynamic anchorage and direct anchorage to prosthesis provide a static attachment to help reattaching extensor mechanism to proximal tibial prosthesis. Protecting the extensor mechanism reconstruction till it attaches through fibrosis along with the surrounding soft tissue structures are crucial to prevent quadriceps lag. Hence, knee bending and quadriceps strengthening will be delayed for six weeks until then the knee is protected with the help of long knee brace. Re-attached gastrocnemius flap could lead to protective muscle spasm of plantar flexors and if not mobilised early it may create plantar flexors contracture. Thus, achieving/maintaining dorsi flexion of the ankle joint in the early post operative period is crucial for appropriate weight bearing. Mobilisation of knee joint and quadriceps strengthening could be started after six weeks; however, therapist must consider that immobilisation of the knee joint in long knee brace leads to severe restriction of patella mobility. Unless adequate patella mobility is achieved, especially the medial lateral movement, knee flexion exercises could prevent smooth gliding of patella over the femoral condyle. This will increase strain on the reconstructed patella ligament. Therefore, our institute follows a unique mobilisation protocol following proximal tibia and knee replacement which is depicted in the Table 2. In few cases quadriceps lag could be evident due to patella tendon overstretch/avulsion, this could be due to improper patella mobilisation or forceful knee bending. Fig. 3 a, b and c depicts the patella tendon overstretch.
Mega prosthetic replacement for proximal and total femoral resection:
Resection of proximal femur and prosthetic replacement may be done for proximal femur tumor or as a part of total femoral resection and reconstruction. Partial or complete loss of joint capsule and dynamic stabilisers of hip joint during tumor resection may leave the hip joint vulnerable to dislocation. This may get potentiated with certain combination movements, if these joint movements are allowed beyond a certain limit. This restriction largely depends upon the surgical approach. Postero-lateral approach being most common in the LSS of this site, hip rotations, especially internal rotation and, flexion more than 600 and adduction of the hip joint needs to be prevented up to 6 weeks [4,7]. These movement restrictions could be achieved using hip abduction pillow/brace and de-rotation splint. Before the patient gets discharged from the hospital, training them for bed transfer, supine to standing, standing to supine and sitting in a chair/commode becomes paramount important in the early phase of rehabilitation. Knee joint mobilisation must be started early either by the edge of bed with hip joint well supported or in side lying with pillows between legs. Any restriction of knee joint range would adversely affect the overall function since hip joint function of the ipsilateral leg has already been compromised. Early FWB ambulation could be started from post operative day one initially with walker, then with walking stick. Later most of them would be trained to walk without any walking aid. Total femoral resections may require more intense rehabilitation with additional emphasis on knee strengthening as discussed earlier (Table 2). Patients may life long need to use walking aids in view of the fact that large motor loss in these cases.
Mega prosthetic replacement for proximal humeral resections:
Proximal humerus and the shoulder girdle are the third common place for primary bone sarcomas [4]. Despite endoprosthetic replacements for functional shoulder girdle structures, such as reverse glenoid prosthesis, are available, lack of muscular structures post excision and damage to the axillary nerve often prevent their use. Frequently, the proximal end of the humerus is replaced with the endoprosthesis and suspended by the remaining muscles and soft tissue structures by suturing around the proximal end of the prosthesis. The objective of the procedure is to achieve a stable shoulder to facilitate good elbow and hand function.
To prevent the weight of the endoprosthesis and the limb acting on the newly formed pseudo joint, shoulder sling and elbow pouch are provided for 4 – 6 weeks. Early post operative rehabilitation consists of elbow and hand range of motion (ROM) and strengthening exercises within pain limit. Again these exercises must be performed in supine position only to avoid undue stress on the shoulder. After six weeks, shoulder joint limited ROM exercise in the form of pendular movements and vigorous strengthening of shoulder girdle, elbow and hand complex should be commenced. Additionally, postural correction must be included in the rehabilitation program.
Biological reconstructions
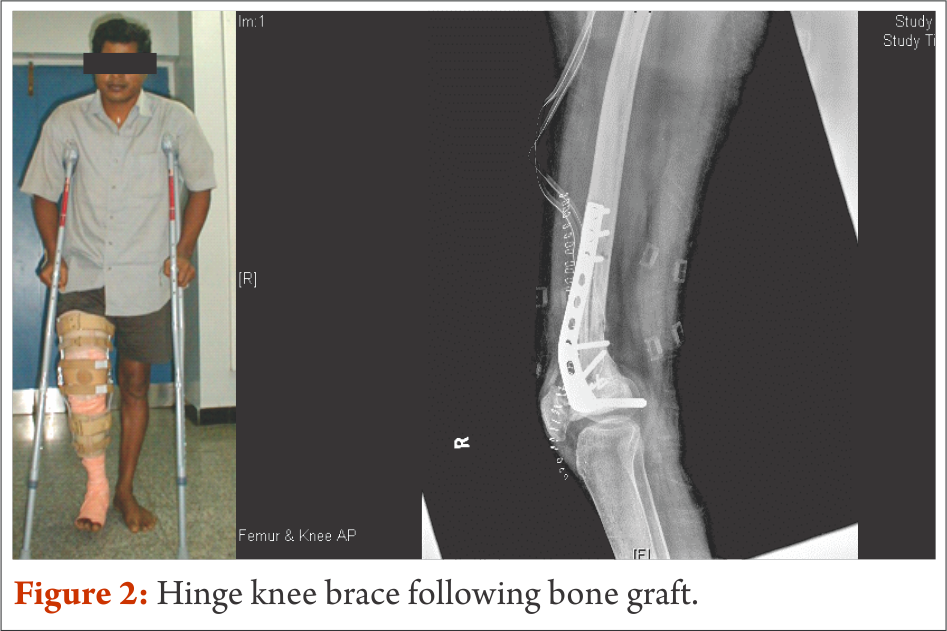
Wherever feasible, biological reconstructions are preferred over endoprosthetic implants in order to provide a stable and permanent solution for reconstruction of defects after tumor resection. However, rehabilitation following biological reconstructions needs careful considerations regarding weight bearing and joint mobilization. Utmost importance to surgical notes and communication with operative surgeon is of prime importance. Early joint mobilisation is the key to prevent joint stiffness and functional loss; nevertheless, often protective functional braces may be required to prevent damage. For example, curettage and bone grafting of the lower end of femur close to the joint demands hinge knee brace to avoid varus and valgus stress. Strengthening exercises also could be started early with functional knee brace (Fig. 4).
Patients are taught to walk non-weight bearing with brace generally from post operative day one with the help of axillary crutches up to 8 weeks. Once, osteosynthesis is confirmed through radiological evaluation, progressive weight bearing walking could be started. FWB walking and complete joint range and strength are expected to be achieved by the end of 3 months to 4 months.
conclusions
Although limb salvage surgery for primary malignant tumours have achieved commendable advancement in surgical techniques and endo-prosthetic design and manufacturing, without optimal and timely peri and post-operative physical rehabilitation, achieving the desired quality of life outcome may not be feasible. This paper has highlighted few important rehabilitation principles and we have summarised rehabilitation protocol for specific area. Most of the oncology resection and reconstruction vary from one individual to another even in one particular site and needs tailor made rehabilitation protocol; nevertheless, this summary will be a guide for necessary foundation to design individual rehabilitation program.
References
1. Chopra BK. Health related quality of life studies in Indian patients after limb salvage surgery: Need of the hour. Med J Armed Forces India. 2013 Jul;69(3):209–10.
2. Ottaviani G, Robert RS, Huh WW, Palla S, Jaffe N. Sociooccupational and physical outcomes more than 20 years after the diagnosis of osteosarcoma in children and adolescents. Cancer. 2013;119(20):3727–36.
3. Frieden RA, Ryniker D, Kenan S, Lewis MM. Assessment of patient function after limb-sparing surgery. Arch Phys Med Rehabil. 1993 Jan;74(1):38–43 6p.
4. Oren R, Zagury A, Katzir O, Kollender Y, Meller I. Principles of rehabilitation after limb-sparing surgery for cancer. In: Musculoskeletal Cancer Surgery [Internet]. Springer; 2001 [cited 2014 Sep 3]. p. 583–93. Available from: http://link.springer.com/chapter/10.1007/0-306-48407-2_36
5. Bekkering WP, Vliet Vlieland TPM, Fiocco M, Koopman HM, Schoones JW, Nelissen RGHH, et al. Quality of life, functional ability and physical activity after different surgical interventions for bone cancer of the leg: A systematic review. SurgOncol. 2012 Jun;21(2):e39–47.
6. Mei J, Zhu X-Z, Wang Z-Y, Cai X-S. Functional outcomes and quality of life in patients with osteosarcoma treated with amputation versus limb-salvage surgery: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014 Nov;134(11):1507–16.
7. Shehadeh A, Dahleh ME, Salem A, Sarhan Y, Sultan I, Henshaw RM, et al. Standardization of rehabilitation after limb salvage surgery for sarcomas improves patients’ outcome. HematolOncol Stem Cell Ther. 2013 Sep;6(3–4):105–11.
8. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010 Jul;42(7):1409–26.
9. Singh F, Newton RU, Galvão DA, Spry N, Baker MK. A systematic review of pre-surgical exercise intervention studies with cancer patients. SurgOncol. 2013 Jun;22(2):92–104.
10. Silver JK. Cancer Prehabilitation and its Role in Improving Health Outcomes and Reducing Health Care Costs. SeminOncolNurs. 2015 Feb;31(1):13–30.
11. Silver JK. Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. SeminOncolNurs. 2015 Feb;31(1):13–30.
(Abstract Full Text HTML) (Download PDF)
Like this: