Role of Image Guided Interventions in Orthopaedic Oncology
Vol 1 | Issue 2 | Sep – Dec 2015 | page:25-29 | Himanshu Pendse[1*], Aniruddha Kulkarni[2], Manish Agarwal[2]
Author: Himanshu Pendse[1*], Aniruddha Kulkarni[2], Manish Agarwal[2]
[1]P.D Hinduja Hospital Veer Savarkar Marg, Mahim, Mumbai, India
Address of Correspondence
Dr. Himanshu Pendse
P.D Hinduja Hospital Veer Savarkar Marg, Mahim, Mumbai, India.
Email: himanshu.pendse@gmail.com
Abstract
Interventional radiology has grown in leaps and bounds specifically in field of orthopaedic oncology. The procedures have advantage of being minimally invasive and have a much better postprocedure rehabilitation. These procedures can be broadly classified as Non-vascular and Vascular interventions. Common non-vascular procedures comprise of image guided biopsies, radiofrequency ablations, vertebroplasty and khyphoplasty. Vascular procedures comprise of Embolotherapy and Sclerotherapy. Isolated Limb Infusion (ILI), MR-HIFU (MRI-guided High Intensity Focused Ultrasound), Cryoablation are few of the more recent advances in the field of interventional radiology. Each procedure has its own indications, contraindications and safety profile. The current article provides an overview of these procedures and there clinical importance.
Keywords: Interventional radiology, orthopaedic oncology.
Introduction
Image guided interventions or otherwise popular as the field of interventional radiology comprise of procedures which are performed with the help of an imaging modality such as fluoroscopy, USG, CT or MRI. With the rising preference for minimal invasive therapies, these procedures enjoy an increasing scope in the patient management. Other than elective procedures, interventional radiology proves invaluable for managing medical and surgical emergencies. In recent years, interventional radiology is playing a vital role in the field of orthopedics and orthopedic oncology. This article will give an outline of the common procedures performed by an interventional radiologist in orthopedic oncology. These procedures can be broadly classified as Non-vascular and Vascular interventions.
Non-Vascular Interventions
Common non-vascular procedures comprise of image guided biopsies, radiofrequency ablations, vertebroplasty and khyphoplasty.
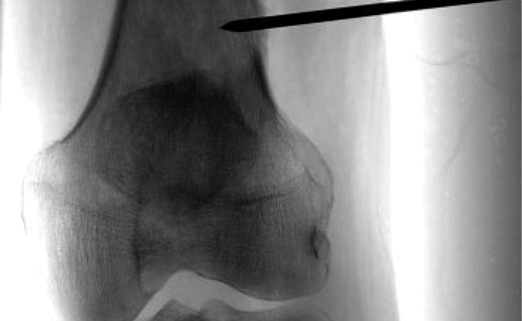
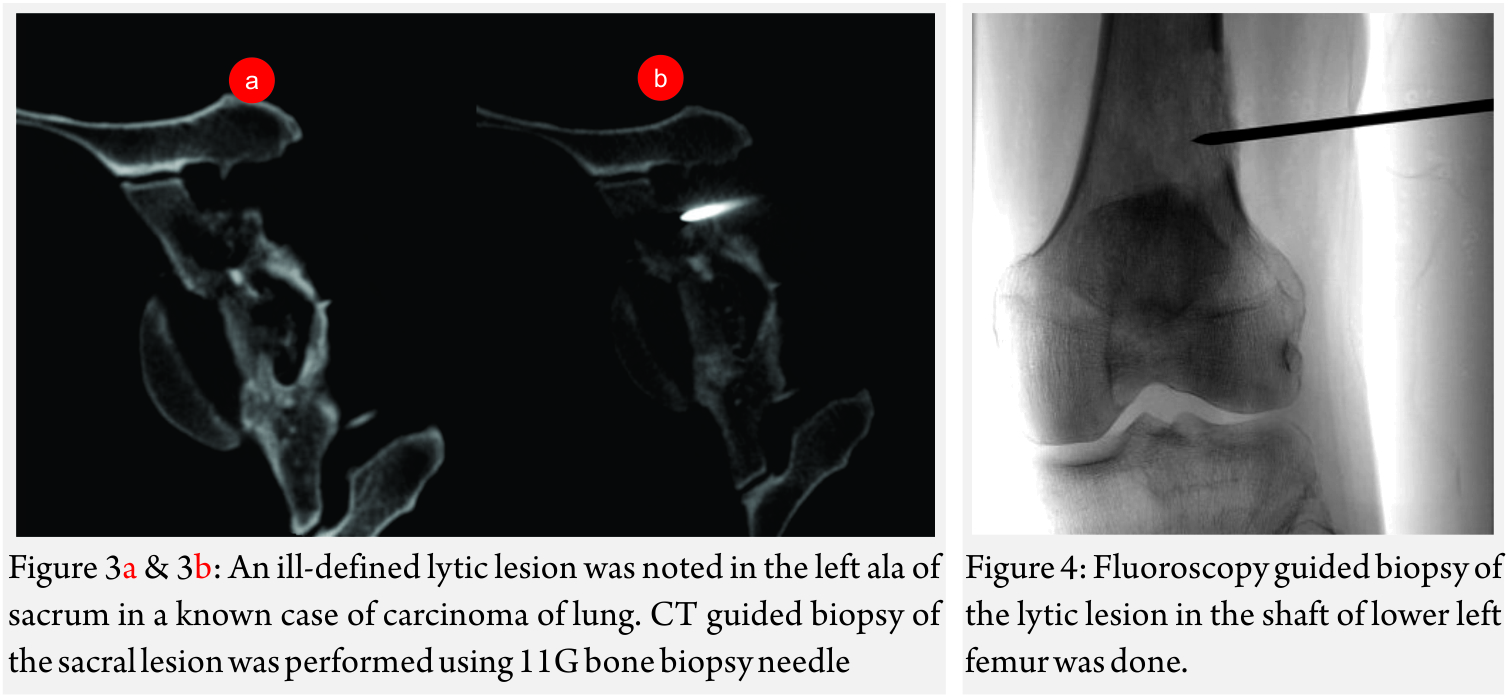
Image guided biopsies and fine needle aspiration cytology (Fig 1A and B, 2A and B, 3A and B and Fig 4):
This is emerged as a safe, faster and accurate tool for getting a diagnosis from a musculoskeletal lesion. It has safer and has better patient tolerance as compared to conventional open biopsy [1].
Indications
1. Determine whether lesion is benign or malignant
2. Obtain material for microbiological examination in a suspected infective lesion
3. Determine status of a lesion in a patient with known primary
Contraindications:
Known coagulopathy, Pregnancy (for CT-guided procedures)
Special considerations
It is imperative to determine a safe route for biopsy, which comes in the excision and/or radiation field for treating the primary lesion. Classic “no-touch” lesions like myositis ossificans in evolving stage; subchondral geodes etc should be identified on imaging and prevented from being biopsied. These lesions appear aggressive on histology, causing radiological and pathological discrepancies.
Equipment and Techniques
Fluoroscopy, USG, CT or MRI have been used for image guidance for a biopsy. CT scan is most often used for biopsy of bone lesions [2]. The main advantage of CT being precise visualization of trajectory of the needle. thus preventing damage to the important structures like the neurovascular bundle.
USG is preferred for superficial bone lesions with a targetable soft tissue component. It is preferred in patients where radiation exposure has to be avoided [3].
Fluoroscopy is a good modality for biopsy of large lesions. In some cases, the lesion is visible only on MRI. In such cases, MRI-guided biopsy is indicated.
The procedure is generally performed under local anaesthesia with light sedation when necessary. The patient lies prone for vertebral biopsy. For any other bone, the position depends on the site of the lesion. Compartmental anatomy greatly influences the needle approach, and such knowledge is critical for preventing unnecessary surgery and loss of limb function. It is important to stress that the biopsy trajectory should be through the tissues that would be excised or comes in the radiation field to prevent recurrence along the biopsy tract.
Generally, an 11-15G Trephine Bone Biopsy Needle is used to target bone lesions without significant soft tissue component. For a FNAC, a 22 G needle is generally used. The yield is better for a larger needle. In case of vertebral biopsy of the dorsal vertebrae, it is advisable to use a 13G needle instead of an 11G needle due to the thinner pedicles of the dorsal vertebrae. In lesions with a significant soft tissue component, an 18G co-axial biopsy set with a co-axial needle and a semi-automated or automated gun would be a good choice. In a circumscribed lytic lesion, it is prudent to use an 11/13G bone biopsy needle as an anchor. Through this, the 18G biopsy needle is passed to obtain soft tissue cores. MRI guided biopsies need special MRI compatible needles. Sampling should be performed by fine-needle aspiration (FNA) along with Core Needle Biopsy as many interventional radiologists feel that both the techniques have a complementary role.
Complications
Pain, bleeding, infection, biopsy tract recurrence and bone fracture are the common complications. Instilling gelfoam torpedos in the biopsy tract can arrest bleeding from biopsy of hypervascular metastasis. Post-procedural Care: Patient may be advised to take painkillers if pain is significant Antibiotic cover is not necessary post-biopsy. Biopsy Yield and Outcomes: This is an important issue for bone biopsies. It is important to take enough samples, especially when benign disease is suspected. Several studies have shown higher accuracy of core needle biopsy (89.7%-96%) to fine needle aspiration cytology (64%-88%) [4]. Biopsy of bone lesions with soft-tissue components (93%, 89%) has higher diagnostic accuracy than biopsy of lytic lesions (85%, 71%) [5]. Also, in case of cystic lesions, biopsy from the wall of the cystic lesion has better yield than aspirating fluid [6]. Infections have been reported to have a high diagnostic yield of 80% to 90% on aspiration [7]. Diagnostic yield has been attributed to larger lesion size and larger specimen length [8].
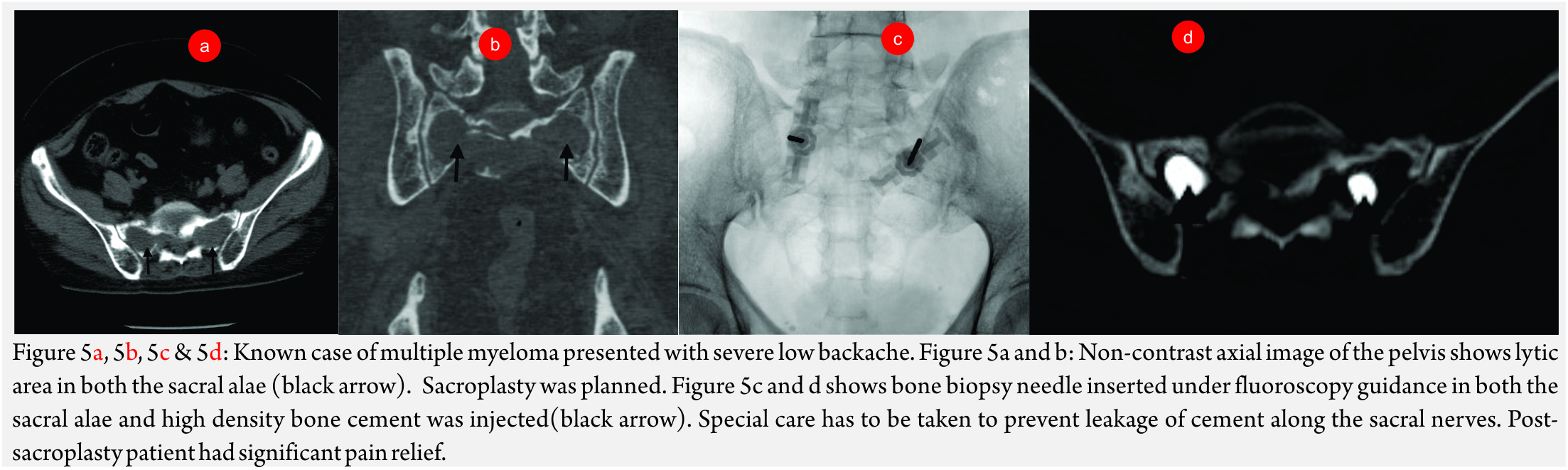
Cementoplasty
This involves injecting bone cement in the weakened weight-bearing bones to relieve pain. The procedure is usually named after the bone, which is treated. For example, acetabuloplasty when acetabular cementoplasty is done and sacroplasty (Fig 5A, B and C) when sacrum is treated.
Vertebroplasty and Khyphoplasty
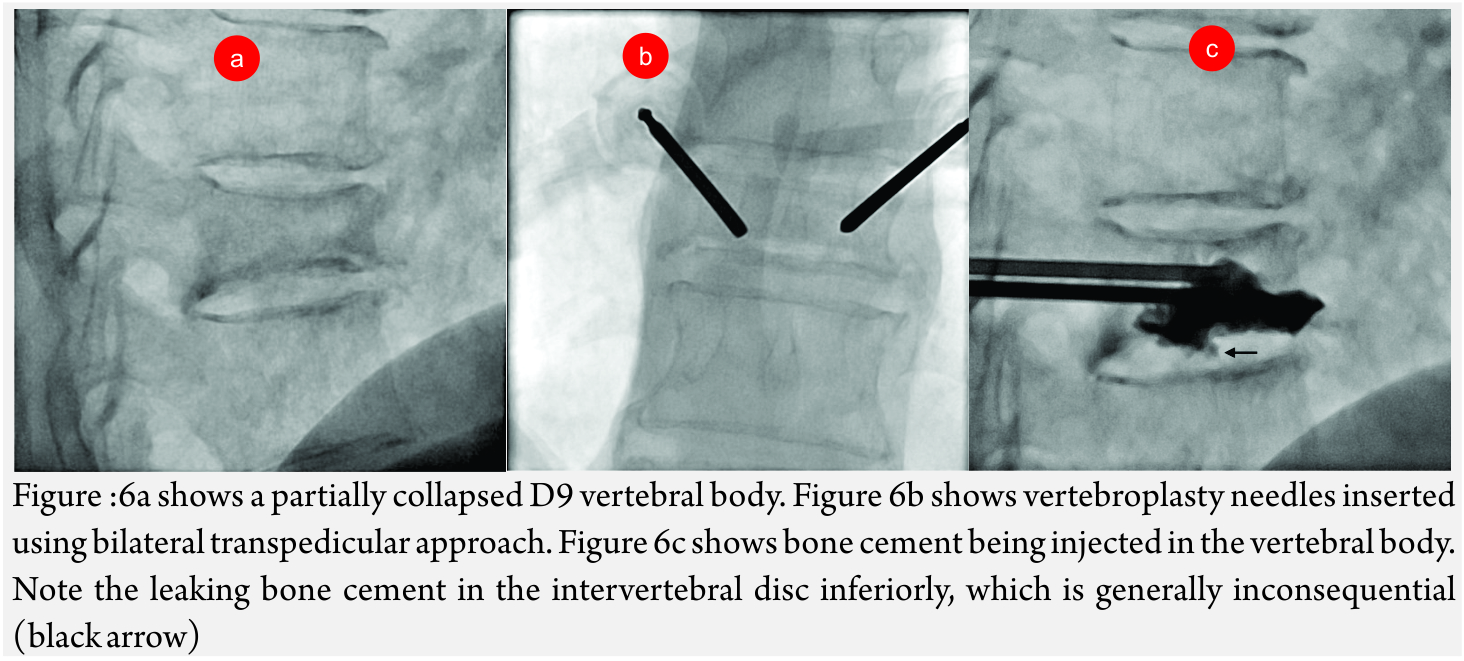
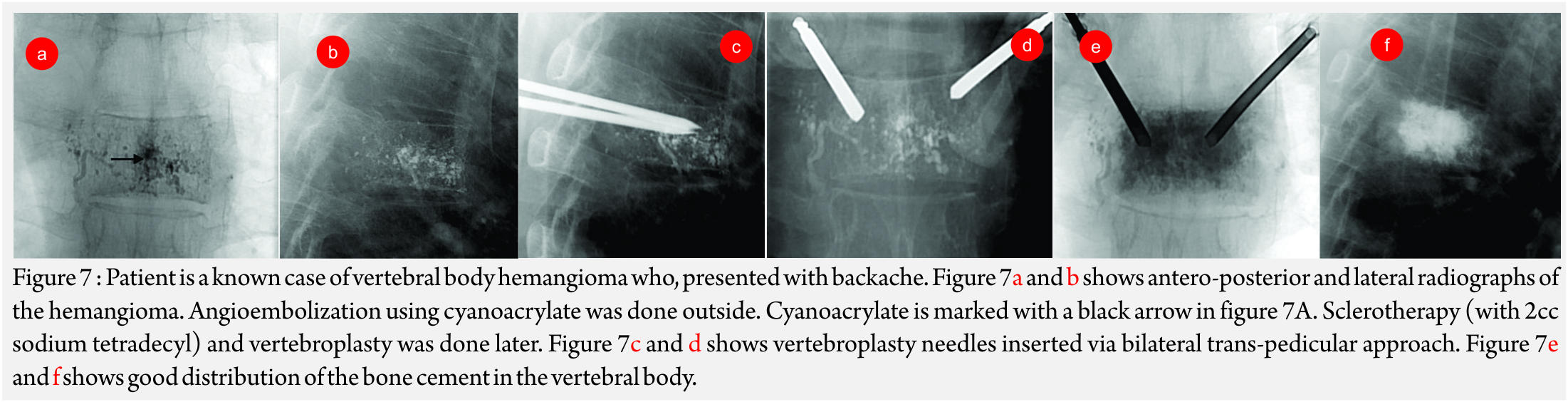
Vertebroplasty and khyphoplasty, also know of vertebral augmentation techniques, involve injection of bone cement in the vertebral body to relieve pain and restore the height of the vertebral body [9]. Indications: Main indication of this procedure is to treat painful vertebral compression fractures which have failed conventional medical therapy. Most common cause is fracture due to primary osteoporotic disease. Others causes like steroid-induced osteoporosis (Fig 6A- C), metastatic disease, compression due to myeloma, hemangioma (Fig A-F) etc are also an indiction for these procedures [10].
Contraindications: Absolute contraindications are uncorrectable coagulopathy, spinal infection, vertebral infection and allergy to bone cement.
Special Considerations: Few of the following situations when present, should alert the interventional radiologist to take extra care during the procedure [11]-
1. Disruption of posterior cortex- increased risk of cement leaking into the spinal cord
2. Vertebra plana or marked loss in the height of the vertebral body
3. Epidural extrusion of tumor
4. Narrow central canal
Pre-procedure assessment and Imaging-
Pre-procedure history and clinical assessment is very important. Pain score before the procedure should be recorded.
Point tenderness at the spinous process should be elicited and correlated with the collapsed vertebra on imaging. A conventional spinal radiograph would be sufficient for this preliminary assessment. A lower limb neurological exam should also be performed. Routine laboratory investigations like complete blood count and coagulation profile should be done.
MRI is the test of choice for pre-procedure evaluation. It shows the exact site of marrow edema, the size of the spinal canal, the course of the exiting nerve roots and allows evaluation of epidural extension of tumor. At the same time, it will also help to make a definitive diagnosis on the etiology of the lesion. CT proves useful to evaluate the integrity of the posterior vertebral cortex.
Technique: Procedure is generally done in prone or oblique position. This position will facilitate extension of the fractured segments [12]. Local anesthesia with or without sedation is required. General anesthesia should be avoided as a conscious patient can alert the clinician about any new symptoms during the procedure.
Routine pre-procedure antibiotic coverage in form of 1gm Cefazolin is given.
Approach: Generally, an 11 G needle is preferred for lower dorsal and lumbar vertebrae while a 13 G needle may be used for upper dorsal vertebrae. It is important to remember the orientation of the vertebral pedicles as one goes down the spine. Generally, two approaches are used to enter the vertebral body- the transpedicular approach and the parapedicular approach. The transpedicular approach has an advantage of protecting the nerve roots and the paravertebral tissue due to the long intraosseous course. The first step is to align the affected vertebral body in such a way that the spinous process is in the midline, the vertebral edges overlap and the pedicle is in the centre of that half of the vertebral body. The entry point is marked on the skin at about 10O’Clock position with respect to the pedicle that will be traversed. Lignocaine is infiltrated at that site and a small skin nick given with an 11 No blade. Needle is inserted from the planned entry point. For transpedicular approach, it is most important to keep the needle, lateral to the medial margin and superior to the inferior margin of the pedicle. This takes care that the needle is in the confines of the pedicle. The needle position could be checked on AP and lateral view on fluoroscopy. Once the needle reaches the posterior margin of the vertebral body, it should be further advanced till the anterior third of the vertebral body in lateral view and till the midline on AP view. Unipedicular approach would suffice if the cement crosses to the opposite side. Otherwise, a bipedicular approach is required [13]. For parapedicular approach, the vertebral body is directly entered and thus the needle is kept lateral to the lateral margin of the pedicle.
Cement Injection for Vertebroplasty:
The cement commonly used is Polymethyl Methacrylate (PMMA). It comes in powder form with a solvent. The powder and solvent are mixed and left for few minutes. The cement is instilled through the needle when it has toothpaste like consistency. It is important to closely monitor leakage of cement in the spinal canal or along the nerve roots. The endpoints for cement injection include passage of cement beyond the marrow space and cement reaching the posterior quarter of the vertebral body. Mathis and Wong have recommended cement filling to 50-70 percent of the residual volume of the vertebral body [14].
Kyphoplasty: Before injecting cement, khyphoplasty involves an additional step of increasing the size of the vertebral body. This is achieved with a khyphoplasty balloon. Later, a dedicated Vertebroplasty injector system can be used to inject PMMA for khyphoplasty. It is recommended to treat up to 3 vertebral levels at a sitting to prevent the complications arising due to marrow fat embolizations [15].
When fractures with Intraosseous vacuum phenomenon (Kummell disease) is noted, it is important to place the needle as close as possible to the cleft in order to allow the cement to fill the cleft.
Complications: Common complications are pain, hematoma, cement extravasation along the nerve roots. Small amount of extraosseous passage of cement is seen in two-thirds of vertebroplasty. As against this, khyphoplasty has a lower rate since the cavity fills first with subsequent hardening of the cement. Other possible complications are paraspinal abscess, hypotension due to cement and fat emboli, pneumothorax, spinal cord damage due to cement extravasation and worsened pain [16]. The risk of complication is more while treating malignancy-related fractures.
Post-procedure monitoring and Follow-up:
The patient should lie supine for an hour after the procedure. Ideally, patient’s can be discharged later on the same day. NSAID’s can be prescribed for the procedure related pain. Many patients have significant relief of symptoms immediately after the procedure. Patient should be informed to report any sudden onset backache as this may indicate a new fracture. 3-week follow-up post-procedure is generally advised. At every follow-up, clinical and pain score assessment is important.
Radiofrequency Ablation: It involves ablating tissues by sending radiofrequency waves in the patient. Basic mode of killing cells involves generation of thermal energy around the electrode.
Principles [17,18]: The radiofrequency wave consists of an alternating current at high frequency (200 –1,200 kHz).
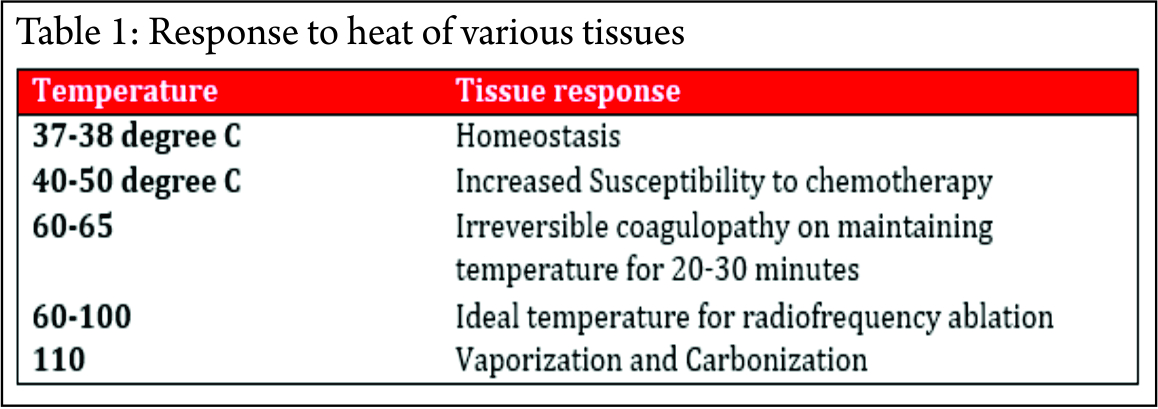
A closed loop circuit consisting of the RF generator, electrode, patient and ground pads (acting as a large dispersing electrode) in series is required. The electrode and the ground pads are active. The RF generator generates the RF current, which enters the tissue to be ablated through the electrode. This current leads to rapid oscillation of the molecules. The patient acts as a resistor. The friction generates heat, which kills the cells. The marked discrepancy between the surface area of the needle electrode and the dispersive electrode causes the generated heat to be tightly focused and concentrated around the needle electrode. The response of the tissue to heat depends on the temperature and the time for which heat was applied (Table 1).
Types of Electrodes: Major categories include monopolar devices, bipolar devices, and coblation devices.
1. Monopolar systems are most commonly used. These require a grounding pad placed on the patient. Their main disadvantage is the formation of aberrant currents, which do not always allow for uniform energy deposition inside the lesion.
They can be divided into single electrode and multi-tined. Single electrodes have the advantage of small caliber but have smaller ablation radius. They can feature hot, cooled-tip, and water-perfused. Multi- tined electrodes increase energy deposition by creating larger zones of ablation and produce better lesion destruction.
2. Bipolar devices do not require a grounding pad, because the current passes through the same or neighboring needles. Advantage is lesser aberrant currents.
Application of Radiofrequency Ablation in Orthopaedic Oncology:
A host of benign and malignant lesions can be treated with radiofrequency ablation. This include- Benign tumors like Osteoid Osteoma, Chondroblastoma, Aneurysmal Bone Cysts and Vertebral hemangiomas
Malignant tumors like spinal metastasis, ablation of soft tissue sarcoma’s to achieve local control.
Other non-oncology applications like radiofrequency neurotomy to treat backache are also practiced.
General Contraindications:
Absolute contraindications to ablation include coagulopathy disorders, skin infection, immunosuppression, and absence of a safe path to the lesion without harming vital organs or structures.
Radiofrequency Ablation for Benign tumors: Among the benign tumors, osteoid osteoma is the commonest benign bone lesion treated with RFA.
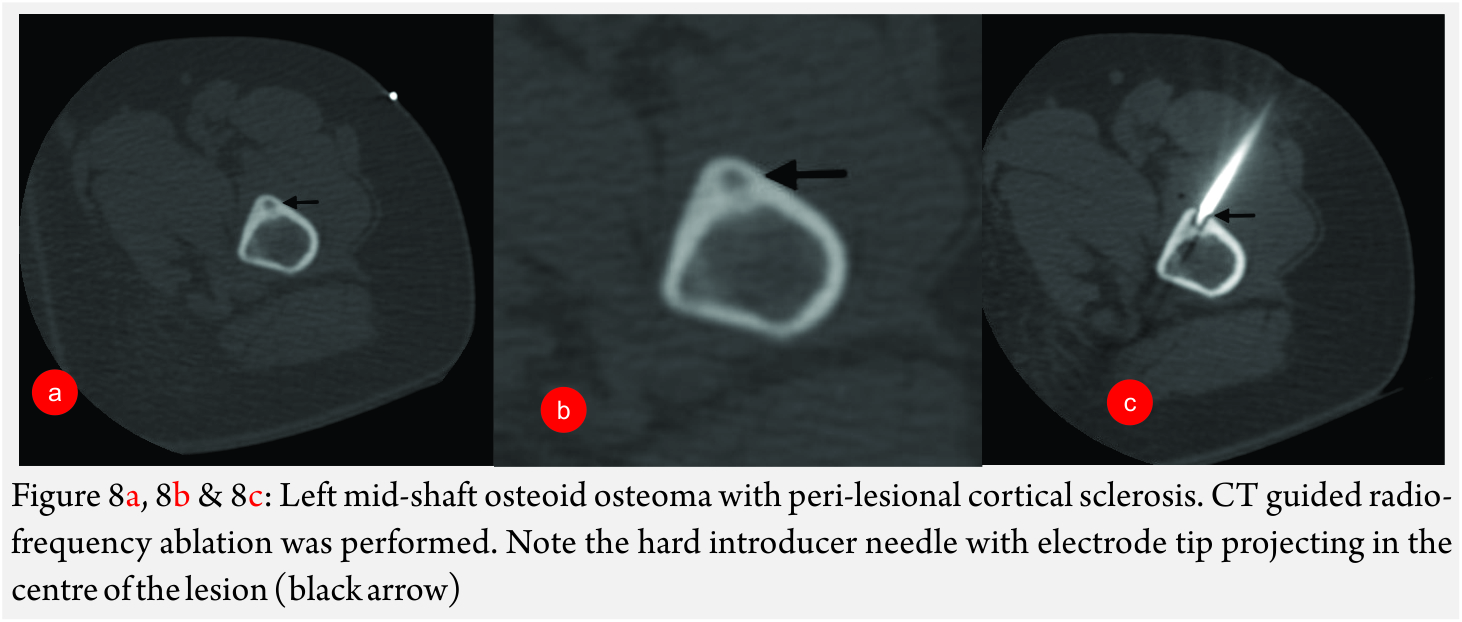
RFA of Osteoid Osteoma [18] (Fig 8A and B): It is a benign lesion, commoner in males (male: female = 4:1). It usually presents in the second decade with nocturnal pain, which is relieved by aspirin or other NSAID’s.
Imaging features: CT is the modality of choice. Typical lesion is seen in the cortex of a long bone with a centrally placed radiolucent nidus and a surrounding sclerotic rim. On contrast enhanced CT study, the nidus shows enhancement.
General technique: Percutaneous radiofrequency ablation is a reliable and effective technique that provides fast, long-lasting pain relief. Bourgault C et al recently published a paper treating 87 patients with osteoid osteoma. In this study, with mean follow-up of 34 months, the success rate for first-line treatment was 89.6% and it was 97.5% for second-line treatment. The recurrence rate was 10.4%.
RFA is generally done under CT guidance. Typically, the skin entry is achieved with a bone biopsy needle, which is advanced till the cortex. In cases where there is significant sclerosis, this thick bone is drilled using a bone drill. Once the lesion enters the lesion nidus, biopsy of the lesion is taken. Further, the RF electrode is inserted through the bore of the biopsy lesion into the lesion nidus. Before, ablation is commenced; the biopsy needle is withdrawn to prevent inadvertent heating of the surrounding tissues due to heat propagation via the needle. Otherwise, the biopsy needle can be exchanged for a hard introducer with a distal insulated tip over a K-wire. The presence of intact cortex around the lesion produces oven effect which leads to better ablation of the lesion.
RFA of other benign tumors: Condroblastoma, an epiphyseal tumor has been treated with RFA as an alternative to surgery. Rybak et al describes 17 patients with chondroblastoma for whom RFA was used as the primary treatment modality. On median follow-up of 41.3 months, 12 out of 17 patients showed complete relief of pain [19].
Aneurysmal bone cyst (ABC) has been treated by RFA of the epithelial lining followed by cementoplasty analogous to curettage and bone grafting. There have been reports where ablation of the ABC wall has been achieved using radioactive material [20].
There are some published reports on the treatment of other benign bone conditions such as enchondroma, eosinophilic granuloma, bone haemangioma and giant cell tumors.
RFA of Malignant tumors: This is usually done for pain relief with a palliative intent. In case of large tumors, compressing nerves, RFA can be performed to debulk the tumors. As RFA destroys cells and theoretically forms a cavity, cementoplasty can be performed along with the RFA to provide tensile strength [21, 22].
Embolotherapy for Musculoskeletal tumors
Transarterial embolization has been practiced for many years in both benign and malignant musculoskeletal tumors. The main purpose of these procedures is to reduce blood supply of the tumor, avoiding non-target embolization [23]. Embolization can be pre-operative embolization, serial embolization or palliative embolization. Pre-operative embolization aims to occlude as a much as possible, helping the surgeon to have a relatively bloodless field. Serial embolization aims to decrease to size of the tumor. This can relieve the patients of the symptoms like pain and in some, can make the patient fir for surgery. Palliative, as the name suggests aims to relive the patients of the symptoms and to improve the quality of life.
Technique: Generally, a pre-procedure cross-sectional imaging is helpful to see the extent and overall vascularity of the neoplasm. A pre-procedure assessment of platelet count, International Normalized Ratio (INR) and creatinine levels is necessary. Initially, a diagnostic angiogram is done to define the supplying vessels following which embolization is performed.
Gelfoam is the embolization material of choice for pre-operative embolization. In cases where serial embolization is required, particulate agents like polyvinyl alcohol (PVA) or embospheres is used. Coils are used where parent vessel occlusion is required or when protection of distal vasculature is necessary.
Embolotherapy in Benign Musculoskeletal Tumors: Embolization is generally performed for benign tumors like Giant Cell Tumor, Aneurysmal Bone Cyst, Vertebral Hemangiomas, Osteoblastomas and arteriovenous malformations.
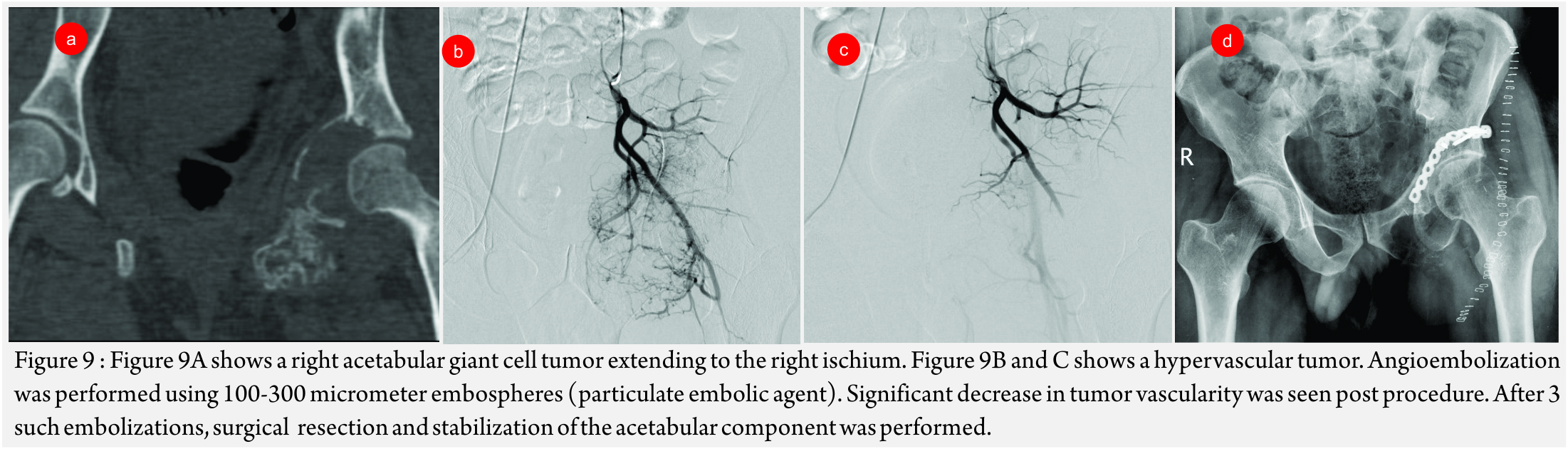
Giant Cell Tumors (GCT’s)(Fig 9 A – D):
GCT’s radiographically appear as expansile lytic lesions in the metaphyseal region reaching the endplate. These have significant vascularity and are associated with significant blood loss.
Sacral GCT’s are especially associated with significant peri-procedure blood loss and post-surgical morbidity. In small operable GCT’s , embolotherapy aims to pre-operatively devascularize the tumor while in cases large sacral GCT’s, it aims to reduce the bulk of the lesion. It can also decrease the associated pain due to compression of the nerve roots [24].
Post-procedure increase in ossification of the lesion is a sign of favourable response to embolization.
In a series of 18 patients of GCT’s, managed with embolization, follow of 26 years showed durable response in 50% of patients with local recurrence rates of 31% in 10 years and 43% in 15-20 years [25].
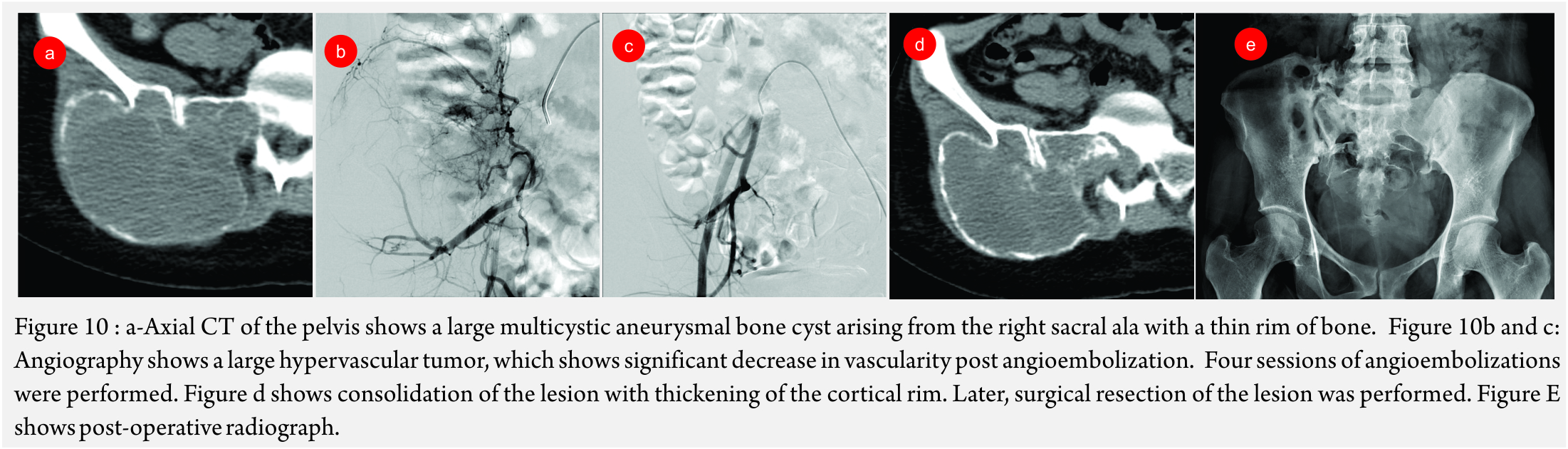
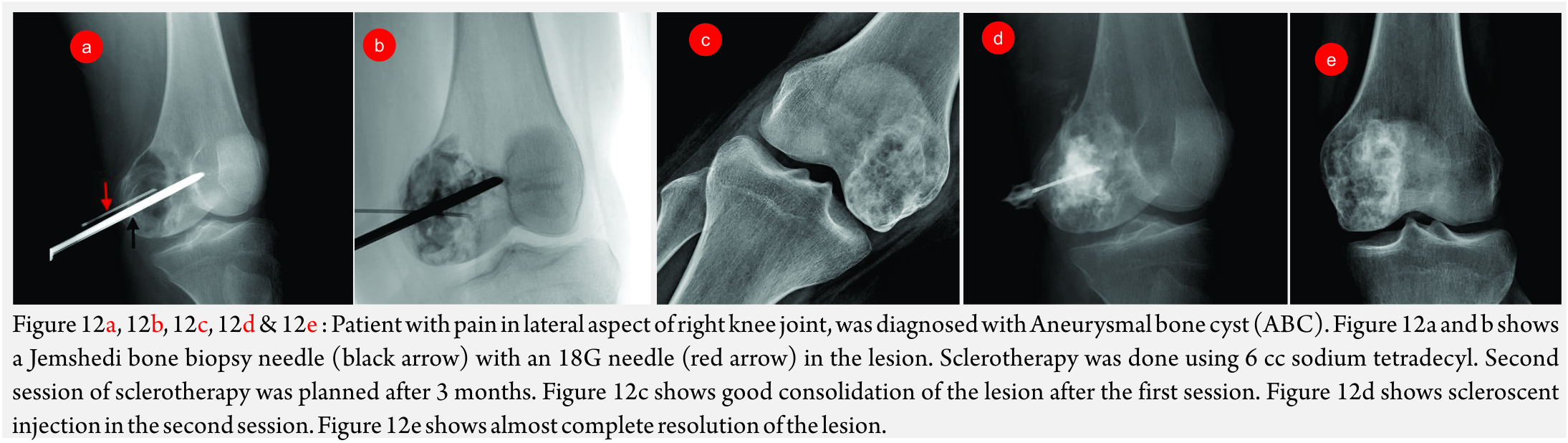
Aneurysmal Bone Cyst (ABC) (Figure 10A and B): Though, curettage and resection are the primary treatment modalities of choice, embolization has been performed in recurrent ABC’s and as a pre-operative measure to reduce blood loss [26].
Vertebral Hemangiomas: Surgery is the modality of choice to treat vertebral hemangiomas causing cord compression or any neurogenic deficit. In these cases, pre-operative embolization serves as an adjuvant to surgery by reducing blood loss[27].
Arteriovenous Malformation of Bone:
Tranarterial embolization can prove as a useful treatment for AVM’s of bone. Direct puncture of the hemangioma with percutaneous embolization has also been attempted to control hemorrhage [28].
Other benign tumors like cervical spine osteoblastomas have been treated by embolization as a adjuvant to surgery.
Embolotherapy in Malignant Musculoskeletal Tumors
Metastases: Hypervascular metastases especially from thyroid and renal carcinomas are treated with embolization. The main intent is palliative and aims to reduce pain and other symptoms that may arise due to compression of the surrounding structures [7]. In 16 cases of renal cell carcinoma metastases bleeding was significantly reduced post embolization [29].
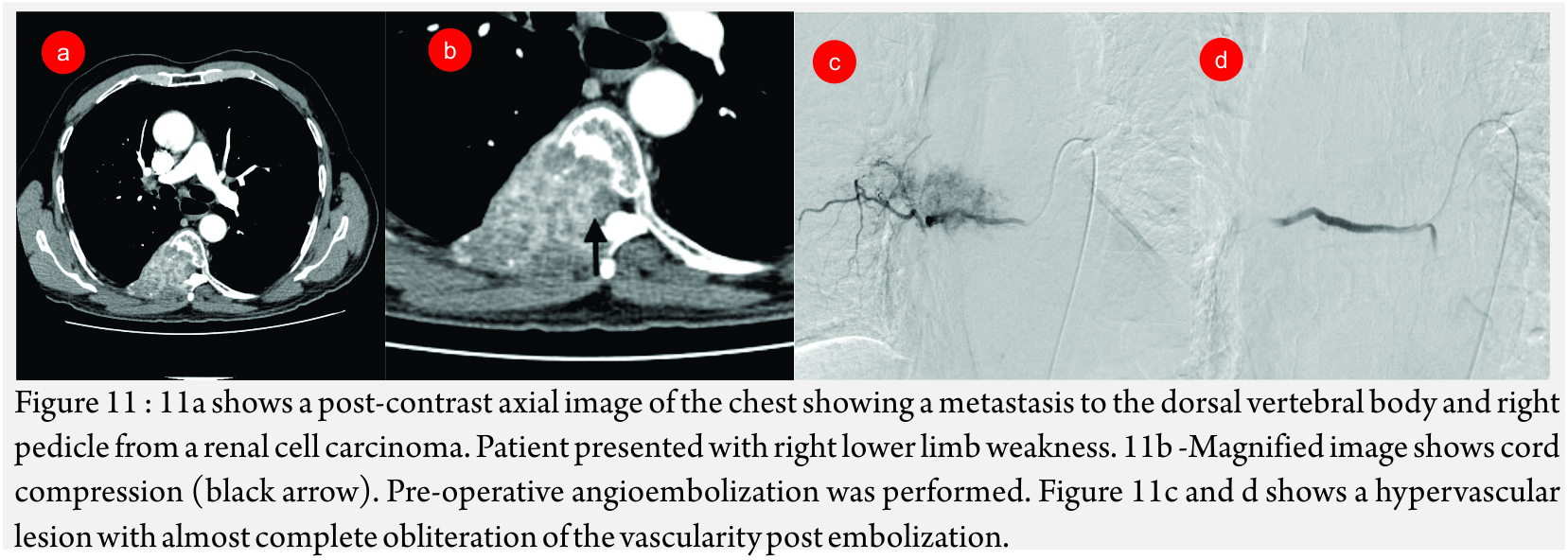
Metastatic spinal tumors (Fig 11A, B and C): Hypervascular spinal and pelvic tumors are treated with embolization as a pre-operative measure to decrease blood loss or as a palliative procedure to relieve symptoms.
Sclerotherapy of Bone Lesions
This involves instilling a scleroscent inside a bone tumor (especially a cystic bone tumor) to damage the lining endothelium, which eventually results in thrombosis.
Sclerotherapy using polidocanol has been attempted in treating Aneurysmal bone cysts (ABC) with encouraging results. In a paper written by Rastogi et al, the author has treated 72 cases of ABC’s with sclerotherapy with satisfactory result in more than 97% [70] of patients [31]. Significant reduction in size of the lesions has been documented. In hypervascular ABC’s, sclerotherapy can be preceded by embolization.
Percutaneous treatment of Vascular Malformation
Though congenital vascular anomalies are not bone lesions, these commonly present as a swelling and are referred to an orthopedic surgeon.
International Society for the Study of Vascular Anomalies (ISSVA) classifies congenital vascular anomalies into vascular tumors and vascular malformations.
Vascular tumors are hemangiomas, which are seen in infancy and childhood. They are further classified into infantile hemangioma, congenital hemangiona, Kaposiform hemangioendothelioma and tufted angiomas. Congenital hemangiomas are classified as Rapidly Involuting Congenital hemangiomas (RICH) and Non- Involuting Congenital hemangiomas (NICH) [32].
Vascular malformations can present anytime in life. They are classified as low-flow vascular malformations and high-flow vascular malformations.
Low-flow vascular malformations are namely venous malformations, lymphatic malformations or mixed while high flow vascular malformations are arterio-venous malformations and arterio-venous fistulas.
Diagnosis: These conditions are diagnosed on the age of presentation, the changes in the lesion over time (progression or regression) and some key imaging features.
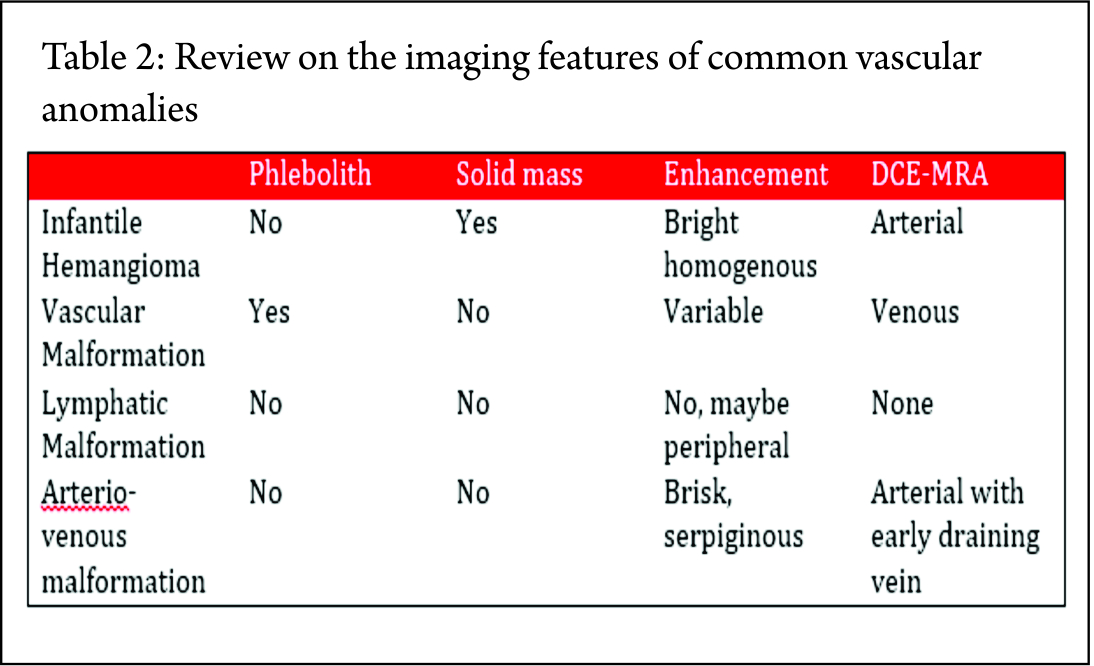
Dyanamic Contrast MR Angiography (DCE_MRA) is the investigation of choice (33). Table 2 provides a review on the imaging features of few of the common vascular anomalies-
Treatment of Vascular tumors
Vascular tumors generally involute over time and eventually regress on follow-up. In cases of high flow lesions, endovascular angioembolization can be attempted to relieve symptoms or to decrease the vascularity before surgery.
Treatment of Vascular Malformations:
Low-flow vascular malformations are most commonly treated by percutaneous sclerotherapy.
Percutaneous Sclerotherapy (Fig 12A, B, C, D, E and F)
This involves injecting a scleroscent in the malformation, which leads to necrosis of the endothelium and subsequent thrombosis.
Contraindications: Atrial septal defects and pulmonary hypertension are absolute contraindications [34].
Technique for venous malformation
The procedure is performed under high quality digital subtraction angiography imaging with road-mapping technique.
The method of sclerotherapy depends on the angioarchitecture of the lesion. Vascular malformations are classified into sequestrated, non-sequestrated and mixed varieties. Sequestrated lesions do not have any communication with the deep venous system, making injecting scleroscent in the lesion safe. The non-sequestrated lesions have communication with the deep venous system while mixed lesions have both the features [35].
Procedure is generally performed under local anaesthesia. General anaesthesia with endotracheal intubation is required for lesions involving the oro-naso-laryngeal pathway or when severe pain is expected during scleroscent injection. Torniquent may be tied to decrease the venous outflow for malformations involving the extremities. Good hydration is necessary to counter scleroscent induced hemolysis.
Procedure: The lesion is punctured using 22G scalp vein needle or 22G spinal needle. Contrast injection is done to evaluate the angioarchitecture. In non-sequestrated lesions, scleroscent is injected into the lesion. For non-sequestrated lesions, outflow tract is occluded with a balloon catheter or glue or ablated with Nd-YAG Laser [36,37].
The commonest used scleorscent is sodium tetradecyl sulphate (Setrol). It is injected as foam. For radioopacity, it is mixed with oil-based solution like Lipiodol or non-ionic contrast. Not more than 0.5ml/kg should be injected at a time with a maximum permissible dose of 20ml [38].
Other scleroscents used are sodium morrhuate, bleomycin, doxyclycine, OKT-432 and ethanol.
Treatment for lymphatic malformation
These are classified as cystic or channel type. Cystic type is further classified as macrocystic, microcystic or mixed. These lesions present as large collections of lymph, commonly in the neck and the axillary lesions.
Lymphatic malformation is punctured with a needle under ultrasound (USG) guidance and fluid is aspirated with or without a pigtail catheter. Later, the scleroscent is instilled in the lesion. Doxycycline is the scleroscent of choice as it can be injected in large quantities [39].
Post-procedure monitoring:
Tight dressing and painkillers are given post-procedure. The lesion tends to increase in size for a few days due to imflammation. This generally settles with painkillers.
Complications: Pain, infection, hematoma and skin blistering are the general complications. Neuropathy may be seen after ethanol injection. Compartment syndrome is a dreadful complication seen when sclerotherapy is done in closed spaces like the orbit. Hemolysis and hemoglobinuria is seen when large amount of scleroscent is used. Other rare complications are pulmonary edema and acute cardiovascular collapse [40].
Advances in Interventional Orthopediac Oncology
Isolated Limb Infusion (ILI) for Malignant Extremity Bone Tumors:
This is a comparatively new approach of delivering high dose of a chemotherapeutic agent in the limb in which the tumor is present. The main advantage is larger quantity of dose of the chemotherapeutic agent can be administered with less systemic side-effects.
ILB has been attempted for melanoma using Actinomycin-D and for soft tissue sarcoma’s using melphalan and tumor necrosis factor [41].
MR-HIFU (MRI-guided High Intensity Focused Ultrasound) [42]:
This involves focusing high intensity ultrasound beam in the desired tissue to be treated. The ultrasound waves cause heat generation in the tissue with coagulative necrosis. MRI is used to guide the treatment. In orthopedic oncology, MR-HIFU can be used for ablation of painful bone metastasis and local tumor control in infiltrative tumors.
Cryoablation [43]: Like radiofrequecy ablation destroys tissue by heat, cryoablation damages tissue by extreme cooling to sub-zero temperatures. It is indicated for painful bone metastasis and for local tumor control. Use of thermocouples to measure temperature is sometimes indicated to prevent damage to surrounding nerves is some cases.
Conclusion
Interventional Radiology has an ever increasing role in the diagnosis and management of bone and soft tissues tumors. Close co-operation and discussions with the orthopedic oncologist and interventional radiologist is the cornerstone for selection of correct treatment modality and optimizing response.
References
1. Mankin HJ, Mankin CJ, Simon MA. The hazard of biopsy revisited.
J Bone Joint SurgAm 1996;78:656–63.
2. Choi JJ, Davis KW, Blankenbaker DG. Percutaneous musculoskeletal biopsy. Semin Roentgenol 2004;39:114–28.
3. Ahrar K, Himmerich JH, Herzog CE, et al. Percutaneous ultrasound- guided biopsy in definitive diagnosis of osteosarcoma. J Vasc Interv Radiol 2004;15:1329–33.
4. Kasraeian S, Allison DC, Ahlmann E, et al. A comparison of fine- needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthop Relat Res 2010;468: 2992–3002.
5. Logan PM, Connell DG, O’Connell JX, et al. Image-guided percutane-
ous biopsy of musculoskeletal tumor: an algorithm for selection of
specific biopsy techniques. AJR Am J Roentgenol 1996;166:137–41.
6. Jelinek JS, Murphey MD, Welker JA, et al. Diagnosis of primary bone tumors with image-guided percutaneous biopsy: experience with 110
tumors. Radiology 2002;223:731–7.
7. White LM, Schweitzer ME, Deely DM, et al. Study of osteomyelitis: utility of combined histologic and microbiologic evaluation of percutaneous biopsy samples. Radiology 1995;197:840–2.
8. Wu JS, Goldsmith JD, Horwich PJ, et al. Bone and soft tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy. Radiology 2008;248:962–71.
9. Phillips FM. Minimally invasive treatments of osteoporotic vertebral compression fractures. Spine 2003;28:S45-53.
10. Stallmeyer MJB, Zoarski GH, Obuchowski AM. Optimizing patient selection in percutaneous vertebroplasty. J Vasc Interv Radiol 2003; 14:683–96.
11. Laredo JD, Hamze B. Complications of percutaneous vertebroplasty
and their prevention. Skeletal Radiol 2004;33:493–505.
12. Teng MM, Wei CJ, Wei LC, et al. Kyphosis correction and height restoration effects of percutaneous vertebroplasty. AJNR Am J Neuro- radiol 2003;24:1893–900.
13. Hu MM, Eskey CJ, Tong SC, et al. Kyphoplasty for vertebral compression fracture via a uni-pedicular approach. Pain Physician 2005;8: 363–7
14. Mathis JM, Wong W. Percutaneous vertebroplasty: technical considerations. J Vasc Interv Radiol 2003;14:953–60.
15. Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol 2001;22:373–81.
16. Nussbaum DA, Gailloud P, Murphy K. A review of complications asso- ciated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related web site. J Vasc Interv Radiol 2004;15:1185–92.
17. Rhim H, Goldberg SN, Dodd GD 3rd, Solbiati L, Lim HK, Tonolini M, Cho OK. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001 Oct;21 Spec No:S17-35; discussion S36-9.
18. Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW, Menendez L. Thermal ablation of osteoid osteoma: overview and step-by-step guide. Radiographics. 2009 Nov;29(7):2127-41.
19. Rybak LD, Rosenthal DI, Wittig JC. Chondroblastoma: radiofrequency ablation–alternative to surgical resection in selected cases. Radiology. 2009 May;251(2):599-604.
20. Charles H. Bush Walter E. Drane Treatment of an Aneurysmal Bone Cyst of the Spine by Radionuclide Ablation AJNR 2000 21: 592-594
21. Callstrom MR, Charboneau JW, Goetz MP, et al. Image-guided ablation of painful metastatic bone tumors: a new an effective approach to a difficult problem. Skeletal Radiol. 2006;35:1–15.
22. Fernando Ruiz Santiago, María del Mar Castellano García et al Treatment of bone tumours by radiofrequency thermal ablation Curr Rev Musculoskelet Med. Mar 2009; 2(1): 43–50.
23. Bucheler E, Hupe W, Hertel EU et al. Catheter embolization of Renal Tumors. Rofo 1976;124(2): 134-38.
24. Turcotte RE, Sim FH, Unni KK. Giant cell tumor of the sacrum. Clin Orthop Relat Res 1993;291: 215-21
25. Lin PP, Guzel VB, MouraMF, at al Long-term follow up of patients with giant cell tumor of sacrum treated with selective arterial embolization Cancer 2002;95(6): 1317-25.
26. Guzey FK, Emel , Aycan A, et al Paediatric vertebral and spinal epidural tumors: a retrospective review of twelve cases. Pediatr Neurosurg 2008;44((1): 14-21.
27. Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas . J Neurosurg 1993;78:36-45
28. Resnick SA, Russel EJ, Hanson DH, et al. Embolization of a life-threatening vascular malformation by direct percutaneous transmandibular puncture Head Neck 1992;14(5): 372-9.
29. Van Tol KM, Hew JM, Jager PL, et al. Embolization in combination with radio-iodine therapy for bone metastases from primary differentiated thyroid carcinoma. Clin Endocrinol 2000; 52: 653-9.
30. Sun S, Lang EV. Bone metastases from renal cell carcinoma: pre-operative embolization J Vas Interv Radiol 1998;9: 263-69.
31. S. Rastogi, M. K. Varshney, V. Trikha, S. A. Khan, B. Choudhury, R. Safaya, J Bone Joint Surg Br September 2006 vol. 88-B no. 9 1212-1216
32. Mulliken JB, Glowacki J. Classification of pediatric vascular lesions. Plast Reconstr Surg 1982;70(1):120–1.
33. Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol 2010;40(6):895–905.
34. Dompmartin A, Blaizot X, Theron J, et al. Radio-opaque ethylcellulose- ethanol is a safe and efficient sclerosing agent for venous malforma- tions. Eur Radiol 2011.
35. Mendonca DA, McCafferty I, Nishikawa H, et al. Venous malformations of the limbs: the Birmingham experience, comparisons and classification in children. J Plast Reconstr Aesthet Surg 2010;63(3): 383–9.
36. Holt P, Burrows P. Interventional radiology in the treatment of vascular lesions. Facial Plast Surg Clin North Am 2001;9(4):585–99.
37. Siniluoto TM, Svendsen PA, Wikholm GM, Fogdestam I, Edström S. Percutaneous sclerotherapy of venous malformations of the head and neck using sodium tetradecyl sulphate (sotradecol). Scand J Plast Reconstr Surg Hand Surg. 1997 Jun;31(2):145-50.
38. Burrows PE, Mason KP. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol 2004;15(5):431–45.
39. Burrows PE, Mitri RK, Alomari A, et al. Percutaneous sclerotherapy of lymphatic malformations with doxycycline. Lymphat Res Biol 2008;6(3–4):209–16.
40. Rimon U, Garniek A, Galili Y, et al. Ethanol sclerotherapy of peripheral venous malformations. Eur J Radiol 2004;52(3):283–7.
41. Wray, C. J., Benjamin, R. S., Hunt, K. K., Cormier, J. N., Ross, M. I. and Feig, B. W. (2011), Isolated limb perfusion for unresectable extremity sarcoma. Cancer, 117: 3235–3241.
42. Bio Joo, Park M-S, Lee SH, et al. Pain Palliation in Patients with Bone Metastases Using Magnetic Resonance-Guided Focused Ultrasound with Conformal Bone System: A Preliminary Report. Yonsei Medical Journal. 2015;56(2):503-509.
43. Callstrom, M. R., Dupuy, D. E., Solomon, S. B., Beres, R. A., Littrup, P. J., Davis, K. W., et al (2013), Percutaneous image-guided cryoablation of painful metastases involving bone. Cancer, 119: 1033–1041.
| How to Cite this article: Pendse H, Kulkarni A, Agarwal M. Role of Image Guided Interventions in Orthopaedic Oncology. Journal of Bone and Soft Tissue Tumors Sep-Dec 2015;1(2): 25-33. |
 |

Dr. Aniruddha Kulkarni |

Dr. Manish Agarwal |
(Abstract) (Full Text HTML) (Download PDF)