Radiological Review of Extremity Osteosarcoma
Volume 2 | Issue 1 | Jan-Apr 2016 | Page 13-18 Amit Janu, Nikshita Jain, Shashikant Juvekar, Ashish Gulia.
Author: Amit Janu[1], Nikshita Jain[1], Shashikant Juvekar[1], Ashish Gulia[2].
[1]Dept of Radiodiagnosis, Tata Memorial Hospital, Parel, Mumbai. India.
[2]Orthopedic Oncology, Dept. of Surgical Oncology, Tata Memorial Hospital, Parel, Mumbai, India.
Address of Correspondence
Dr Ashish Gulia, MS (Ortho), Mch (Surgical Oncology)
Associate Professor, Orthopedic Oncology, Dept. of Surgical Oncology, Tata Memorial Hospital,
Mumbai – 400012, India.
Email: aashishgulia@gmail.com
Abstract
The paradigm shift in the overall outcomes of osteosarcoma is multi factorial. Be it introduction of chemotherapy, introduction of better
imaging or advances in the technology to produce better prosthesis, the crux lies in the correct and timely diagnosis. Radiology along with
clinical evaluation and histopathological confirmation is an essential part of the three tier system which leads to an accurate diagnosis
which forms the platform to initiate an ideal treatment to have desired oncological and functional outcomes. Plain radiography has been
the age old diagnostic tool which is still as important as was in yester century. Inclusion of high end cross sectional imaging likeCT and
MRI has not only helped in early and price diagnosis but has also proved to be a boon to operative surgeons to stage the disease locally and
plan complex limb salvage strategies. In the present article we have discussed the radiological features of various sub types of osteosarcoma
to help a clinician to assess these complex varieties of lesions.
Keywords: osteosarcoma, radiological assessment.
Introduction
Osteosarcoma is the most common primary nonhematologic bone malignancy and is the most common primary bone malignancy in children. There are various subtypes of osteosarcoma, each with distinct clinical and imaging characteristics and variable survival and an incidence of 0.2 to 0.3 per 100,000/year. Osteosarcomas can be classified as intramedullary (high grade, telangiectatic, low grade, small cell, osteosarcomatosis, gnathic), juxtacortical (parosteal, periosteal, intracortical, high-grade surface), or secondary lesions [1]. Though majority of cases are of conventional high grade intramedullary osteosarcoma accounting for 75%- 90%, it is important to differentiate them from other low grade and (low grade central and parosteal osteosarcoma) and intermediate grade osteosarcoma (Periosteal osteosarcoma). An understanding of the systematic imaging approach helps to more accurately diagnose these lesions and direct effective treatment. This article provides an organized approach to analyzing and subtyping of osteosarcoma based on radiographs and guiding the referring physician if any further imaging is warranted as there is frequently overlap with other benign and malignant entities, creating substantial diagnostic challenges. For accurate diagnosis, it is important to be aware of radiographic and cross-sectional imaging features that allow differentiation of each subtype of osteogenic sarcoma from its mimics. Osteosarcoma is a malignant tumor that is characterized by production of osteoid matrix (immature bone) and variable amounts of cartilage matrix and fibrous tissue [2]. Each subtype of the osteosarcoma exhibit distinct imaging features mimicking different benign and malignant entities, however with critical evaluation of specific features a correct diagnosis can be made. Furthermore, important prognostic information, as well therapeutic options can be evaluated based on imaging.
Conventional osteosarcoma
Conventional intramedullary osteosarcoma is the most common subtype of osteosarcoma, accounting for 75% of all cases. Conventional osteosarcoma is a high-grade neoplasm produces osteoid matrix by the tumour cells centrally within the bone and eventually involves the entire width of the bone. It is often described as amorphous, fluffy, cloud-like, solid, cotton like or ivory like on the plain radiographs. It appears as homogenously increased density within bone and in soft tissues. Approximately 90 % of the osteosarcomas show some degree of osteoid matrix on radiographs [3]. Histologically osteosarcoma is pleomorphic and can produce variable amounts of cartilage, fibrous tissue, or other components. Some osteosarcomas produce more than one type of matrix and depending on the dominant cell type, they can be further subdivided into osteoblastic (50%–80%), fibroblastic-fibrohistiocytic (7%–25%), chondroblastic (5%–25%), telangiectatic (2.5%–12%), or small cell (1%) (4). Most cases of conventional osteosarcoma are seen in second and third decades of life, peaking when patients are aged 10 to 15 years, while they are unusual in patients younger than 6 years or older than 60 years [5]. The imaging characteristics of the various subtypes of osteosarcoma are summarized in Table 1.
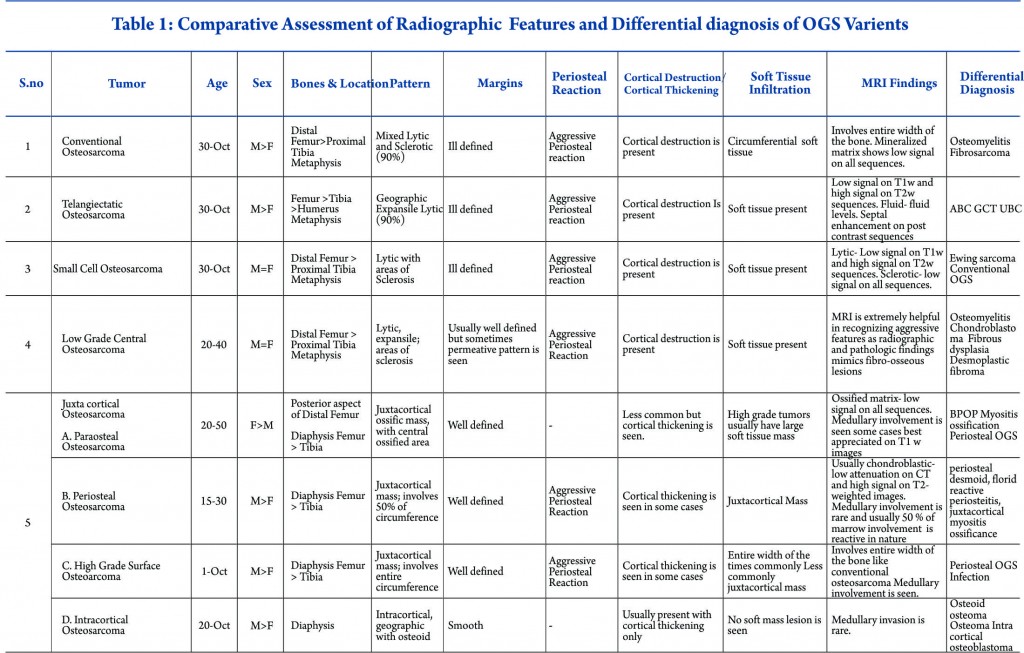
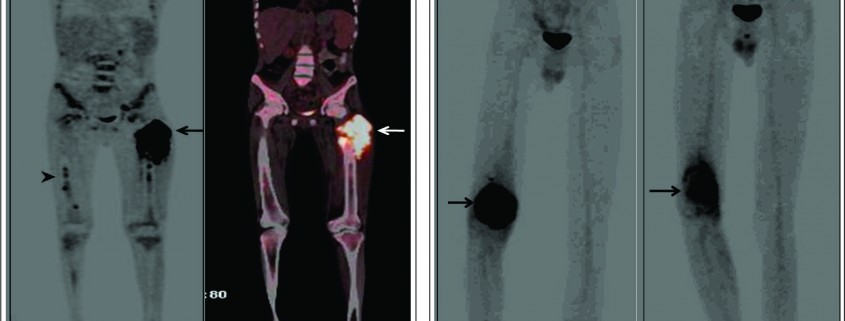
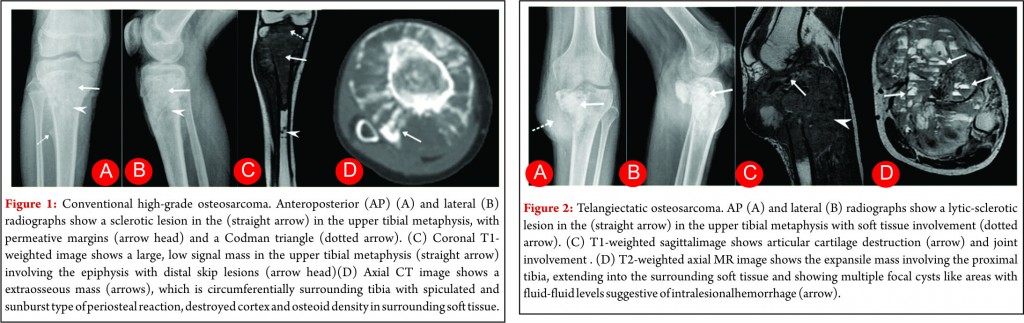
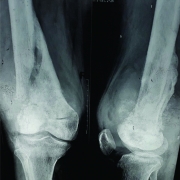
Conventional osteosarcoma most frequently affects long bones (70%–80%), particularly near the knee, in the femur, tibia, and humerus. This lesion originates in the metaphysis, with extension to the epiphysis (seen in up to 80% of MR imaging studies) (Fig. 1C), however initial manifestation in epiphysis alone is extremely rare [6]. Patients with conventional osteosarcoma may present with pathologic fractures. Skip lesions also occur in about 5% of patients. The intraosseous and extraosseous extent of tumor seen in cross sectional imaging should be measured and documented which is vital in preoperative assessment and staging of osteosarcoma. Joint involvement is seen in 19% to 24% of cases and is diagnosed when hyaline cartilage is penetrated and synovial involvement is rarely seen [7]. Radiographic findings are characteristic, osteosarcoma tends to destroy cortex without expanding osseous contours, reflects its aggressive nature with osteoid matrix having a pattern of fluffy opacities, with aggressive periosteal reaction (laminated, hair-on-end, sunburst, or Codman triangle) and with a soft tissue mass in 80-90 % of the cases (Fig.1). Occasionally, the lesions are purely lytic (fibroblastic) or sclerotic (osteoblastic), but most common pattern seen is mixed lytic and sclerotic [8]. MR imaging is the examination of choice for local staging and for planning biopsies or surgery because of superior contrast resolution and multiplanar imaging. The entire involved bone should be scanned to evaluate for skip metastases. The lytic areas appears low signal on T1-weighted images and high signal on T2-weighted images, whereas the mineralized matrix appears low signal on both T1- weighted (see Fig.1C) and T2-weighted images. The T1- weighted images gives vital information regarding the anatomical extent of the marrow involvement, invasion into epiphysis and skip lesions. Treatment includes chemotherapy followed by wide surgical resection and limb salvage or amputation. Local recurrence is high if there has been a pathologic fracture. Staging work-up should include a non contrast chest CT and a whole-body bone scan. Approximately 15% to 20 % of patients present with radiographic metastases. Most common site for metastasis are lungs followed by other bones. All high-grade osteosarcoma are treated with a multimodality management. The standard sequence include a multiagent chemotherapy (Doxorubicin, cisplatin, high-dose methotrexate, etoposide and ifosphamide) followed by wide surgical excision of the primary tumor which is followed by adjuvant chemotherapy. Addition of chemotherapy has dramatically improved the overall outcomes of extremity osteosarcomas from a mere 20% to 60–70%.
Telangiectatic osteosarcoma
Telangiectatic osteosarcoma accounts for 2 %–7% of all osteosarcomas cases and most commonly occurs in the 1st and 2nd decades of life. Telangiectatic osteosarcomas are located in the metaphysis of long bones and show asymmetric expansion, geographic lysis of bone, with an aggressive growth pattern (ill defined margins) with cortical destruction, minimal sclerosis and soft tissue mass [9, 10]. On pathological analysis, it shows dilated cavities filled with blood and septa and a small solid mass or a rim that contains high-grade osteosarcomatous cells. Commonly it appears low attenuation mass on computed tomography (CT), low signal on T1-weighted MR imaging, and high signal on T2-weighted MR imaging with fluid-fluid levels are seen in up to 90% of these lesions (Fig. 2). The imaging and pathologic features of these lesions may be confused with those of aneurysmal bone cysts, giant cell tumor, metastases and chondroblastic conventional osteosarcoma [11, 12]. The presence of thick, nodular, solid tissue within or around the cystic spaces, best seen on contrast-enhanced MR imaging along with aggressive pattern of growth and presence of matrix mineralization, is also helpful in making the diagnosis. Matrix mineralization in these lesions may be subtle on radiographs and it is better seen on CT. Imaging also helps to guide biopsy of the viable tumour areas. It is of utmost importance that these tumors must not be confused with other differentials and a biopsy should be performed before embarking on any form of definitive surgical procedure. The staging work up and management of telangiectatic osteosarcoma is similar to that of a conventional osteosarcoma.
Small-cell osteosarcoma
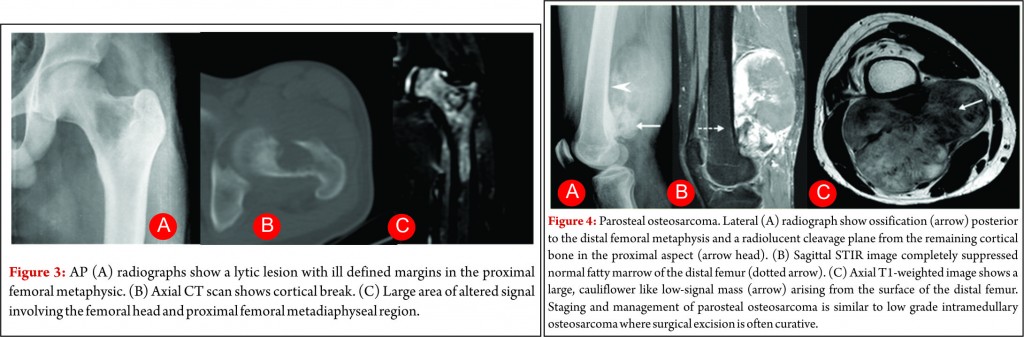
Small cell osteosarcoma is a distinct but rare subtype of conventional osteosarcoma which represents approximately 1- 4% of osteosarcoma cases. It most often affects patients in the 2nd and 3rd decades of life. The pathologic characteristics may be mistaken for Ewing sarcoma or primitive neuro- ectodermal tumor because its cells are small and have round and hyperchromatic nuclei, but cells of small cell osteosarcoma lack uniformity and consistently produce osteoid [13]. These lesions are most commonly seen in the metaphysis like conventional osteosarcoma, but they can be seen purely in the diaphysis in 15% of cases [14, 15]. Small-cell osteosarcoma is an intramedullary, permeative lytic lesion that is associated with cortical destruction, aggressive periosteal reaction, and soft tissue mass (Fig. 3) [16]. Differential diagnosis includes Ewing sarcoma, lymphoma, and conventional osteosarcoma. Although osteoid matrix is typically seen, purely lytic lesions may occur in up to 40% of cases. The prognosis is poor than that of conventional osteosarcoma. Some centres modify the chemotherapy like that of Ewing sarcoma due to presence of round cells but no standard concensus exist [14]. Over all staging and management of these tumors is also similar to other high grade osteosarcoma.
Low-grade central osteosarcoma
Low-grade central osteosarcoma is uncommon variant of conventional osteosarcoma also referred to as well differentiated or sclerosing osteosarcoma [20]. The mean age of presentation is slightly older and occurs in 3rd or 4th decade of life [21]. The radiologic and pathologic findings simulates those of fibrous dysplasia and benign fibro-osseous lesions often resulting in erroneous radiographic and histologic diagnosis. The presence of aggressive features like cortical destruction, permeative pattern or a soft tissue mass is helpful in differentiation of low-grade central osteosarcoma from benign fibro-osseous lesions which are better seen on CT and MRI. These cases are usually staged with a chest radiograph only. Surgery forms the main cornerstone of the management. Chemotherapy is not warranted and these are treated with wide surgical excision. The outcomes are usually excellent with wide excision, however intra-lesional resection and curettage can result in high local recurrences and transformation of initial lesion into high grade sarcoma as well [22].
Juxtacortical osteosarcoma
Juxtacortical or surface osteosarcoma refers to originating from the surface of bone and accounts for 4% to 10% of all osteosarcomas. It is usually associated with the periosteum and cortex with variable medullary canal involvement. These lesions are further divided into parosteal, periosteal, high grade surface, and intracortical osteosarcomas because of different radiological and histological findings.
Parosteal osteosarcoma
Parosteal osteosarcoma is the most common type of juxtacortical osteosarcoma originates from the outer layer of the periosteum, accounting for 65% of juxtacortical osteosarcomas and typically manifesting in the third and fourth decades [23]. The lesion is slightly more commonly seen in women. The tumor usually occurs in the metaphysis of long bones and posterior aspect of the distal femur is the most frequent site. Pathologically, it is usually a low grade tumour with extensive osteoid matrix and minimal fibroblastic stroma with occasional areas of cartilage are seen. At radiography, the classic appearance is a lobulated, cauliflower like, juxtacortical centrally dense ossific mass, separated by radiolucent cleavage plane with adjacent normal cortex in its early stages (approximately 30% of cases at radiographs and in 65% of cases at MRI) [24, 25]. This cleavage plane refers to the periosteum interposed between the normal cortex and the tumor mass. Cortical thickening with a relative lack of aggressive periosteal reaction may also be seen (Fig.4). The ossified matrix is predominantly low in signal intensity on both T1- and T2-weighted images (Fig.4), while unmineralized soft-tissue mass larger than 1 cm3 is predominantly high in T2 signal intensity. High signal intensity indicates high grade tumour [24]. Medullary cavity invasion may be seen in 8% to 59% of cases on MR imaging, and although the prognosis of these patients of these patients is controversial, knowledge of this invasion helps in complete surgical resection. Prognosis in patients with parosteal osteosarcoma is excellent, with a 10-year survival rate of 80%. High-grade foci warrant adjuvant chemotherapy. The main differential diagnosis includes myositis ossificans, osteochondroma and periosteal chondroma. Apart from trauma history, the gradual ossification of the lesion from the periphery toward the center of the mass and no attachment to the cortex is a characteristic radiographic finding of myositis ossificans [26]. Osteochondroma have corticomedullary continuity between the tumor and the underlying medullary canal which lacks in parosteal osteosarcoma [27].
Periosteal osteosarcoma
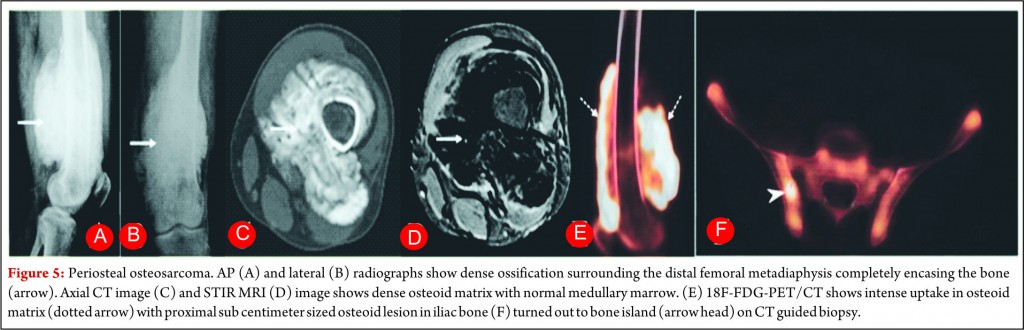
Periosteal osteosarcoma is the second most common type of juxtacortical osteosarcoma originates from the inner germinative layer of periosteum, accounts for 25% of juxtacortical osteosarcomas and usually presents in second and third decades with slight male preponderance [28, 29]. Pathologically, it is predominantly cartilaginous with small areas of osteoid, intermediate cytologic grade distinctly lower than that of conventional osteosarcoma but higher than that of parosteal osteosarcoma. Periosteal osteosarcoma characteristically occurs in diaphysis or metadiaphysis and usually involves 50% of the osseous circumference (Fig.5). Common radiographic findings include a broad-based mass on the surface of the bone, with cortical erosions, cortical thickening and periosteal reaction. Though medullary extension can occur, it is still rare and reactive marrow changes can occur in 50% of the cases [28, 29]. Periosteal reaction is seen as perpendicular low signal intensity areas on all MR sequences arising from the inner cortex to the outer margin of the tumor (Fig.5). Pathologically tumours are chondroblastic and they usually appear low attenuation on CT and high signal on T2-weighted images. Perpendicular periosteal reaction is seen as rays of low signal intensity on all MR imaging sequences. Prognosis of patients is better than conventional osteosarcoma but worse than parosteal osteosarcoma. Treatment consists of wide local excision with limb salvage. Perisoteal osteosarcomas are intermediate grade tumors and are staged like high grade osteosarcomas. A wide surgical resection is mandatory but role of chemotherapy is controversial.
High-grade surface osteosarcoma
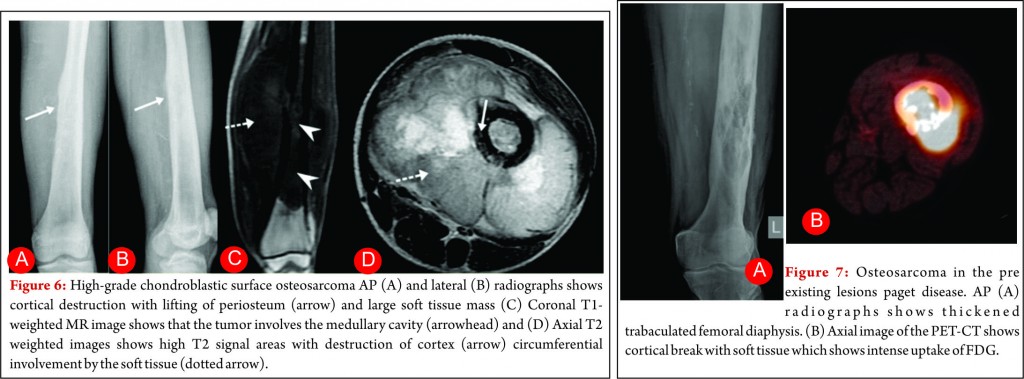
High-grade surface osteosarcoma is least common type of osteosarcoma and accounts for 10% of juxtacortical osteosarcomas. It usually manifests in second and third decade of life. Pathologically, it is high grade like conventional osteosarcoma. Radiologically, it affects the diaphysis or metadiaphysis of the long bones, involves the entire circumference of the bone and may invade the medullary cavity [30, 31]. These tumors are staged and treated like other high grade osteosarcoma.
Intracortical osteosarcoma
Intracortical osteosarcoma is a rare type of osteosarcoma that arises from the cortex and is most commonly seen in second decade. Radiologically, they affect diaphysis long bones and have a geographic lytic area with variable amounts of mineralized osteoid. The lesions may also have smooth margins and variable cortical thickening with common differential diagnosis for this condition includes osteoid osteoma or osteoblastoma. Medullary invasion is rare.
Secondary osteosarcoma
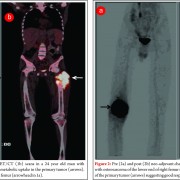
Although conventional osteosarcoma and secondary osteosarcoma are histologically indistinguishable, diagnosis is made on the basis of typical radiographic appearances in the pre existing lesions such as MFH or paget disease (Fig.7) or secondary to radiation. The prognosis for these patients is usually poor.
Osteosarcomatosis (multifocal osteosarcoma)
Osteosarcomatosis is a condition characterized by multiple intraosseous osteosarcomas believed to represent rapidly progressive multicentric metastatic disease. It accounts for 3% to 4% of all osteosarcomas. Most of these patients have a multiple radiographic lesions and pulmonary metastatic disease. Mean survival for these patients is less than 1 year
Conclusion
Osteosarcoma is the most common primary bony malignancy in children’s. The radiologic appearances vary over a wide spectrum and may be mimicked by various benign and malignant lesions still each subtype have often characteristic radiographic features and are suggestive of the specific diagnosis most of the time. Perhaps more important, additional cross sectional imaging modalities specifically MR imaging, provide vital information for planning biopsies or preoperative staging in surgical management. Recognition of these imaging features is an important guide for the accurate diagnosis which helps our clinical colleagues regarding the often difficult and complex multimodality treatment of patients with osteosarcoma to improve the clinical outcome.
References
1.White LM, Kandel R. Osteoid-producing tumours of bone. Semin Musculoskelet Radiol 2000;4(1):25–43.
2.Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol 2006;125(4): 555–581.
3.Murphy M.D., Robbin M.R., McRae G.A., et al: The many faces of osteosarcoma. Radiographics 1997; 17: pp. 1205-1231.
4.Mirra JM: Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, Lea & Febiger 1989. 248-438.
5.Huvos AG: Osteogenic sarcoma. In: Bone Tumors: Diagnosis, Treatment and Prognosis. Philadelphia: WB Saunders 1991; 85-156.
6.Ghandur-Mnaymneh L, Mnaymneh WA, Puls S.The incidence and mechanism of transphyseal spread of osteosarcoma of long bones. Clin Orthop 1983; 177:210-215.
7.Schima W, Amann G, Stiglbauer R, et al. Preoperative staging of osteosarcoma: efficacy of MR imaging in detecting joint involvement. AJR 1994; 163:1171-1175.
8.Dahlin DC, Coventy MB. Osteogenic sarcoma: A study of six hundred cases. J. Bone and Joint surgery 1967; 49:101-110.
9. Weiss A, Khoury JD, Hoffer FA, et al. Telangiectatic osteosarcoma: the St. Jude Children’s Research Hospital’s experience. Cancer 2007;109(8):1627– 1637.
10. Murphey MD, wan Jaovisidha S, Temple HT, Gan- non FH, Jelinek JS, Malawer MM. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology 2003;229(2):545–553.
11.Murphey MD, Nomikos GC, Flemming DJ, Gan- non FH, Temple HT, Kransdorf MJ. Imaging of gi- ant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radio- Graphics 2001;21(5):1283–1309.
12.Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical presentation, and im- aging. AJR Am J Roentgenol 1995;164(3):573–580.
13.Sim FH, Unni KK, Beabout JW, Dahlin DC. Osteosarcoma with small cells simulating Ewing’s tumor. J Bone Joint Surg Am 1979;61(2):207–215.
14.Nakajima H, Sim FH, Bond JR, Unni KK. Small cell osteosarcoma of bone: review of 72 cases. Can- cer 1997;79(11):2095–2106.
15.Ayala AG, Ro JY, Papadopoulos NK, Raymond AK, Edeiken J. Small cell osteosarcoma. Cancer Treat Res 1993;62:139–149.
16.Edeiken J, Raymond AK, Ayala AG, Benjamin RS, Murray JA, Carrasco HC. Small-cell osteosarcoma. Skeletal Radiol 1987;16(8):621–628.
17.Finklestein DUB. Osteosarcoma of the jaw bones. Radiol Clin North Am 1970; 8:425-433.
18.Garrington GE, Scofield HH, CornynJ, Hooker SP. Osteosarcoma of the jaws: analysis of 56 cases. Cancer 1967; 20:377-391.
19.Lee YY, Van Tassel P, Nauert C, Raymond AK, EdeikenJ. Craniofaciab osteosarcomas: plain film. CT, and MR findings in 46 cases. AJR 1988; 150: 1397- 1402.
20.Unni KK, Dahlin DC, McLeod RA, Pritchard DJ. Intraosseous well-differentiated osteosarcoma. Cancer 1977;40(3):1337–1347.
21.Andresen KJ, Sundaram M, Unni KK, Sim FH. Imaging features of low-grade central osteosarcoma of the long bones and pelvis. Skeletal Radiol 2004;33 (7):373–379.
22.Kurt AM, Unni KK, McLeod RA, Pritchard DJ. Low-grade intraosseous osteosarcoma. Cancer 1990;65(6):1418–1428.
23.Antonescu CR, Huvos AG. Low-grade osteogenic sarcoma arising in medullary and surface osseous locations. Am J Clin Pathol 2000;114(suppl):S90– S103.
24.Jelinek JS, Murphey MD, Kransdorf MJ, Shmookler BM, Malawer MM, Hur RC. Parosteal osteosarcoma: value of MR imaging and CT in the prediction of histologic grade. Radiology 1996;201(3): 837–842.
25.Campanacci M, Picci P, Gherlinzoni F, Guerra A, Bertoni F, Neff JR. Parosteal osteosarcoma. J Bone Joint Surg Br 1984;66(3):313–321.
26.Kransdorf MJ, Meis JM, Jelinek JS. Myositis ossificans: MR appearance with radiologic-pathologic correlation. AJR Am J Roentgenol 1991;157(6): 1243–1248.
27.Lin J, Yao L, Mirra JM, Bahk WJ. Osteochondromalike parosteal osteosarcoma: a report of six cases of a new entity. AJR Am J Roentgenol 1998;170(6): 1571–1577.
28. Murphey MD, Jelinek JS, Temple HT, Flemming DJ, Gannon FH. Imaging of periosteal osteosarcoma: radiologic-pathologic comparison. Radiology 2004;233(1):129–138. 19.
29.Revell MP, Deshmukh N, Grimer RJ, Carter SR, Tillman RM. Periosteal osteosarcoma: a review of 17 cases with mean follow-up of 52 months. Sarcoma 2002;6(4):123–130.
30.Staals EL, Bacchini P, Bertoni F. High-grade surface osteosarcoma: a review of 25 cases from the Rizzoli Institute. Cancer 2008;112(7):1592–1599.
31.Okada K, Unni KK, Swee RG, Sim FH. High grade surface osteosarcoma: a clinicopathologic study of 46 cases. Cancer 1999;85(5):1044–1054.
| How to Cite this article:.Janu A, Jain N, Juvekar S, Gulia A. Radiological Review of Extremity Osteosarcoma. Journal of Bone and Soft Tissue Tumors Jan-Apr 2016;2(1): 13-18. |