Biological Methods of Reconstruction After Excision of Extremity Osteosarcoma
Volume 2 | Issue 2 | May-Aug 2016 | Page 5-9 | Suman Byregowda1, Ajay Puri1, Ashish Gulia1
Authors: Suman Byregowda[1], Ajay Puri[1], Ashish Gulia[1]
[1] Orthopedic Oncology Services, Department of Surgical Oncology, Tata Memorial Hospital, Mumbai.
Address of Correspondence
Dr. Ashish Gulia
Associate Professor, Orthopedic oncology, Department of Surgical Oncology, Tata Memorial Hospital, Mumbai.
Email: aashishgulia@gmail.com
Abstract
Introduction: The overall survival rates for non-metastatic osteosarcomas have dramatically improved from a mere 15-20 percent to 60-65 percent today. This was possible due a multifactorial improvement in all the disciplines and specifically the advent of multiagent chemotherapy. With an exponential increase in the survival as well as limb salvage procedures, it would be customary to invent cost effective, stable, durable reconstruction options. Various biological and non biological methods are available for reconstruction. In the era of metal and with the advent of growing artificial bones, non biological options appear to be an attractive and easily available option with excellent immediate results but their long term results and complications are debatable. On the other hand the less attractive biological methods are known to provide stable, durable, cost effective reconstruction options. In the present article we discuss various biological reconstruction methods available for extremity osteosarcoma patients, their advantages and disadvantages.
Keywords: Biological reconstruction , Osteogenic sarcoma
Introduction
The era when osteosarcomas of the extremity were treated with only amputations is long past and the advent of multimodality management has completely changed the outcomes of these tumors. With newer chemotherapic agents, modern surgical techniques, better imaging techniques and affordable reconstructive options limb salvage has become the norm resulting in better functional and psychological outcomes The prerequisites for limb salvage include the ability to achieve an oncologically safe margin and ability to reconstruct the limb such that it provide better function compared to an amputation. Today this is possible in more than 95 percent of the patients [1].
Adequate oncologic clearance is paramount and the chosen method of reconstruction should never compromise the amount of resection required. The barriers to limb salvage are encasement of a major motor nerve, major vascular involvement, poorly placed biopsy incisions, uncontrolled infection, displaced pathological fractures and inadequate motors after resection of tumors.
Besides fulfilling the basic pre requisites of limb salvage mentioned above the reconstructive modality chosen should permit an early return to daily activities and be aesthetically acceptable. . The reconstruction must be durable, economically feasible and should have minimum short term and long term complications. A number of reconstructions methods, both biological and non biological are available for the reconstruction of these skeletal defects after resection. The chosen method of reconstruction should be tailored for the individual based on the growth potential, site and amount of resection and functional requirements. This article discusses the biological techniques available for reconstruction of bone defects after resection of an extremity osteosarcoma.
Biological methods available for reconstructions are
A) Allografts
B) Autografts – vascularised and non vascularised
C) Patient’s own sterilized tumor bone
D) Combination of allografts/ sterilized tumor bone and vascularised autografts
E) Distraction osteogenesis with Ilizarov technique
F) Rotationplasty
G) Masquelet technique
Depending on the extent of the resection, the surgical resections can be categorised as Osteo-articular resections and Intercalary resections. Reconstruction after osteoarticular resections is mainly done by megaprosthesis (non biological). If you want to retain joint mobility the biological options available are limited to osteoarticular allografts. Though these maintain bone stock and provide a better attachment for surrounding soft tissue resulting in increased stability of the construct the long term results with osteoarticular allografts are disappointing .Fracture, arthritis, non unions, infections and repeated surgery are not uncommon. Studies have reported 60-70 percent adverse events, overall 5 year survival of 69 % and 79 % for allograft and articulate surface respectively[2,3]. A composite of allograft and prosthesis has been widely used, where allograft helps to maintain the stock and prosthesis provides the articular surface [figure 1]. The functional outcomes with composite reconstruction are comparable with prosthetic reconstruction alone but associated with higher complication like nonunion and fracture. This method can have limited use in selected young patients with expected long term survival and require good bone stock for revision surgeries [4,5,6].
Allografts require sophisticated bone banks for procurement and storage and these are not available in most of the developing countries. Bone donations are not as frequent as other organ donations making procuring of size matched allografts even more challenging. Allografts may also be associated with risk of transmission of disease.
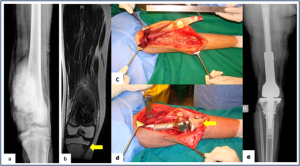
Figure 1: Allograft-prosthesis combination (a,b) Plain radiograph and MRI showing osteosarcoma of distal femur with solitary metastasis to ipsilateral proximal tibia. (c,d) Distal femur and proximal tibia resected with oncological principles, saving the extensor mechanism. Distal femur reconstructed with prosthesis and proximal tibia reconstructed with size matched tibial allograft (arrow). (e) Plain radiograph showing allograft- prosthesis composite
Allograft-prosthesis combination (a,b) Plain radiograph and MRI showing osteosarcoma of distal femur with solitary metastasis to ipsilateral proximal tibia. (c,d) Distal femur and proximal tibia resected with oncological principles, saving the extensor mechanism. Distal femur reconstructed with prosthesis and proximal tibia reconstructed with size matched tibial allograft (arrow). (e) Plain radiograph showing allograft- prosthesis composite
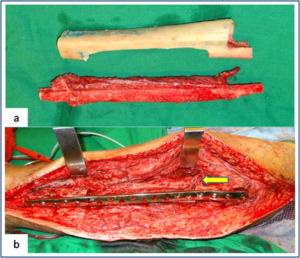
Figure 2: Vasularised fibula + allograft combination (a) Showing vascularised fibula with pedicle and prepared tibial allograft. (b) Allograft and vasularised fibula construct used for the intercalary resection of femur. Arrow showing the microvascular anastomosis between donor and recipient vessels.
Though strut allografts alone can be used for the reconstruction of intercalary defects and knee arthrodesis but studies have shown higher rate of complication like fracture, non union and resorption of grafts. Study by Bus MP et al has demonstrated a complication rate of 76 % and 70 % chance for reoperation due to graft failure. Thus strut allografts alone have limited use and are generally preferred for the upper limb or small defects (< 15cms). To overcome the above complication strut allograft may be combined with vascular fibular grafts [7,8].
Fibula is the most widely used autograft for reconstruction. It can be used as a vascularised or non vascularised graft. Proximal fibular head (with articular surface) has been used to reconstruct the articular surface of proximal humerus and distal radius. While isolated vascularized fibula may be adequate for reconstruction of upper limb defects where weight bearing is not an issue, lower limb reconstructions involving femur or the knee generally require a combination of vascularised fibula with strut allografts [figure 2]. Isolated use of fibula autograft or strut allografts have higher failure rates in large lower limb bone defects [9,10,11]. Small osteoarticular defects (up to 5 cm) like after the resection of distal radius lesions can also be reconstructed with iliac crest autograft. Certain anatomical sites have an inherent advantage and ease for reconstruction. Use of the neighbouring bone in forearm and leg provides a vascularised graft after resection of the radius and tibia. This serves as an easy and effective method of reconstruction. Shifting the distal ulna after an osteotomy at an appropriate level into the defect along with its soft tissue attachment and stabilizing it to the radius proximally and carpal bone distally (wrist arthordesis) provides an excellent method of reconstructing the bone defects after resection of distal radius tumors[figure 3]. This method provides a stable wrist while maintaining forearm rotations (pronation- supination)[12]. Similarly in tibial lesions the fibula is mobilized medially into tibial defect and stabilized. This can be done both, for intercalary resections of the tibia where fibula is shifted after a double osteotomy and distal intrarticular resections where the transposed fibula is stabilized to talus to create an ankle arthrodesis This procedures avoids the requirement of a complex micro vascular procedure, reduces the operative time and also facilitates ease of soft tissue closure as transportation of fellow bone in to the defect will result in volume reduction of the tissues [13].
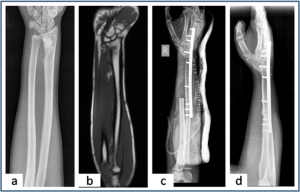
Figure 3: Ulnar translocation (a,b) Plain radiograph and MRI showing the osteosarcoma of distal radius.(c) Immediate post operative radiograph shows the ulna in the distal radius defect and wrist arthrodesis. (d) Union at both radio-ulna and carpal-ulna junction after 3 months of surgery.
Reimplanting sterilized tumor host bone is widely used after intercalary resections. Patients own resected bone is sterilized and used to fill the defect. The resected tumor grafts can be sterilized by various methods like radiotherapy (extra-corporeal radiotherapy), pasteurization, autoclaving, liquid nitrogen and microwave.
This technique has various advantages over the use of allograft. It does not require a bone bank, provides size matched graft (as it has been taken from the same defect) and has no risk of transmitted disease. After resection of the tumor the tumor bearing bone is taken on a separate table and soft tissues are removed under aseptic precautions. Certain soft tissues like ligaments may be retained on the bone graft in order to facilitate reconstruction. Sterilized bones are implanted back in the defect and stabilized with intramedullary nails or plates [figure 4]. Reimplanted bone acts a scaffold for creeping substitution and incorporation. To enhance incorporation and the union at osteotomy sites they can be combined with a vasclarised fibula ( Capanna technique). Puri et all documented a mean union time of 7 months for osteotomy sites and an excellent MSTS score of 29 with extracorporeal radiotherapy [14,15]. To overcome the adverse events like nonunion, fracture and collapse with the use of liquid nitrogen to sterlise tumor bone (fresh frozen autograft), pedical autograft technique was developed. In this technique an osteotomy is done at one end or joint disarticulation done and the whole specimen is treated with liquid nitrogen with other end in continuity with the main bone. It is then stabilized back with internal fixation or athroplasty. As bony continuity is maintained at one end, it is presumed to have early blood flow recovery and faster union and less complication compared with frozen autograft [16,17]. The main drawback of sterilized bones are inadequate mechanical strength resulting in graft fracture and implant failure. To enhance incorporation and to overcome inadequate mechanical strength they can be combined with a vasclarised fibula ( Capanna technique).
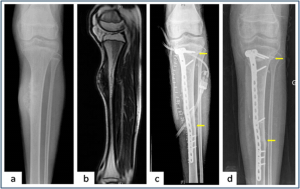
Figure 4: Intercalary resection and extra-corporeal radiotherapy ( a,b) Plain radiograph and MRI showing the periosteal osteosarcoma of tibia. (c) Plain radiograph showing Intercalary resection of the tibia. Resected bone was irradiated and stabilised in the defect with Locking plate. Arrows showing osteotomy sites (d) Union at both metaphysical and diaphyseal osteotomy ( arrows) site after 9 months of follow up.
For surface lesions like periosteal, parosteal and high grade surface osteosarcomas where medullary canal is not involved, bone preserving hemicortical excision may be considered. Meticulous planning with MRI and CT scans are required to obtain adequate margins and preserve good native bone. Computer assisted navigation surgery is advantageous while performing such technically demanding bone preserving surgeries. Various options are available to fill the bone defect after hemicortical excision (sterlised resected bone, strut allografts or small defects can be filled with autografts) [18,19].
Rotationplasty involves converting ankle joint to knee joint by segmental resection and rotating the foot externally to 180 degrees. This is an alternative method for reconstruction especially in children with growth potential where cost constraints may preclude the use of expensive growing prosthesis. This worthies also useful in converting hind quarter or above knee amputations to a functional below knee like amputation in adult patients where conventional resection and reconstructions are not possible due to large or previously inappropriately treated lesions In distal femur and proximal tibia lesions, a segment of involved bone along with knee joint and involved soft tissues is removed only sparing the neurovascular bundle. Here the two segments are connected only with neurovascular bundle. The distal fragment is externally rotated 180 degrees and distal part of femur is stabilized to proximal tibia with appropriate implants, in such a way that the ankle comes to the level of opposite knee joint. In the cases with involvement of whole femur, proximal tibia is articulated with the hip joint with or without use of prosthesis after external 180 dgree rotation. Adequate soft tissue reconstructions and an intense rehabilitation protocol ensures an excellent functional outcome in these cases where the ankle will act like knee joint, dorsiflexion of ankle acts as flexion and plantar flexion acts as extension of knee joint [figure 5]. This procedure can also be used as salvage surgery following infected and failed limb salvage reconstruction. Studies have shown excellent oncological and functional outcome with this procedure. Rotationplasty offers a durable reconstruction option. It is not associated with phantom limb pain or sensations which are common following amputations. The main drawback of the procedure is the cosmetic deformity due to posterior rotated foot [20,21].
Distraction osteogensis using ilizarov method has been used for bone defects in tumor resection. It can be combined with live fibula grafts. The disadvantages are prolonged duration of treatment, high incidence of pin tract infections due to immune compromised state of patient receiving chemotherapeutic agents [22,23]. Due to these complications it is not a popular method and is used rarely.
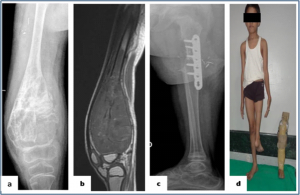
Figure 5: : Rotationplasty (a,b) Plain radiograph and MRI showing the osteosarcoma of the distal femur in a skeletally immature patient. (c) Plain radiography showing the union between femur and tibia after 3 months of surgery. (d) Clinical picture showing rotationplasty patient with externally rotated foot and prosthesis used for the same
Masquelet technique is a two stage procedure for reconstruction of bone defects. In the first stage the defect is filled with bone cement and stabilized. This leads to the formation of a biological membrane over the cement spacer. In second stage procedure the biological membrane is opened, cement spacer removed, filled with cortico-cancellous bone graft and biological membrane sutured to create close content. The procedure was described in children. The ideal time for stage two is between 6 to 8 weeks, though in oncology we wait for completion of adjuvant treatment. Advantage is it makes primary surgery short and rapid uptake of graft due to biological membrane after the second procedure. The disadvantage with procedure is requirement of two surgical interventions [24].
conclusion
Reconstruction following tumor resection is a challenging task. Different biological and non biological methods are available. Selection of a reconstruction procedure should be tailored to the individual patient based on the bone affected, amount of resection, requirement of patient and expertise and infrastructure available at treating centre. Biological methods are more cost effective and provide durable reconstruction options in properly selected extremity osteosarcoma patients.
References
1. Ghert M, Deheshi B, Holt G, Randall RL, Ferguson P, Wunder J et al.Prophylactic antibiotic regimensin tumour surgery (PARITY): protocol for a multicentre randomised controlledstudy. BMJ Open. 2012 Nov 28;2(6).
2. Muscolo DL, Ayerza MA, Farfalli G, Aponte-Tinao LA. Proximal tibia osteoarticular allografts in tumor limb salvage surgery. Clin OrthopRelat Res. 2010 May;468(5):1396-404.
3. Toy PC, White JR, Scarborough MT, Enneking WF, Gibbs CP. Distal femoral osteoarticular allografts: long-term survival, but frequent complications. Clin OrthopRelat Res. 2010 Nov;468(11):2914-23.
4. Donati D, Colangeli M, Colangeli S, Di Bella C, Mercuri M. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin OrthopRelat Res. 2008;466:459–65.
5. Van de Sande MA, Dijkstra PD, Taminiau AH. Proximal humerus reconstruction after tumour resection:Biological versus endoprosthetic reconstruction. IntOrthop. 2011;35:1375–80.
6. Campanacci L, Manfrini M, Colangeli M, Alí N, Mercuri M. Long-term results in children with massive bone osteoarticular allografts of the knee for high-grade osteosarcoma. J PediatrOrthop. 2010 Dec;30(8):919-27.
7. Bus MP, Dijkstra PD, van de Sande MA, Taminiau AH, Schreuder HW, Jutte PC, van der Geest IC et al. Intercalary allograft reconstructions following resection of primary bone tumors: a nationwide multicenter study. J Bone Joint Surg Am. 2014 Feb 19;96(4):e26.
8. Aponte-Tinao LA, Ayerza MA, Muscolo DL, Farfalli GL. Allograft reconstruction for the treatment of musculoskeletal tumors of the upper extremity. Sarcoma.2013;2013:925413.
9. Scaglioni MF, Chang EI, Gur E, Barnea Y, Meller I, Kollander Y, Bickels J, Dadia S, Zaretski A. The role of the fibula head flap for joint reconstruction after osteoarticular resections. J PlastReconstrAesthet Surg. 2014 May;67(5):617-23.
10. Campanacci DA, Puccini S, Caff G, Beltrami G, Piccioli A, Innocenti M, Capanna R. Vascularised fibular grafts as a salvage procedure in failed intercalary reconstructions after bone tumour resection of the femur. Injury. 2014 Feb;45(2):399-404.
11. Hilven PH, Bayliss L, Cosker T, Dijkstra PD, Jutte PC, Lahoda LU, Schaap GR, Bramer JA, van Drunen GK, Strackee SD, van Vooren J, Gibbons M, Giele H, van de Sande MA. The vascularised fibular graft for limb salvage after bone tumour surgery: a multicentre study. Bone Joint J. 2015 Jun;97-B(6):853-61.
12. Puri A, Gulia A, Agarwal MG, Reddy K. Ulnar translocation after excision of a Campanacci grade-3 giant-cell tumour of the distal radius: an effective method of reconstruction. J Bone Joint Surg Br. 2010 Jun;92(6):875-9.
13. Puri A, Subin BS, Agarwal MG. Fibular centralisation for the reconstruction of defects of the tibial diaphysis and distal metaphysis after excision of bone tumours. J Bone Joint Surg Br. 2009 Feb;91(2):234-9.
14. Mottard S, Grimer RJ, Abudu A, Carter SR, Tillman RM, Jeys L, Spooner D. Biological reconstruction after excision, irradiation and reimplantation of diaphyseal tibial tumours using an ipsilateralvascularised fibular graft. J Bone Joint Surg Br. 2012 Sep;94(9):1282-7.
15. Puri A, Gulia A, Jambhekar N, Laskar S. The outcome of the treatment of diaphyseal primary bone sarcoma by resection, irradiation and re-implantation of the host bone: extracorporeal irradiation as an option for reconstruction in diaphyseal bone sarcomas. J Bone Joint Surg Br. 2012 Jul;94(7):982-8.
16. Igarashi K, Yamamoto N, Shirai T, Hayashi K, Nishida H, Kimura H, Takeuchi A, Tsuchiya H. The long-term outcome following the use of frozen autograft treated with liquid nitrogen in the management of bone and soft-tissue sarcomas. Bone Joint J. 2014 Apr;96-B(4):555-61.
17. Shimozaki S, Yamamoto N, Shirai T, Nishida H, Hayashi K, Tanzawa Y, Kimura H, Takeuchi A, Igarashi K, Inatani H, Kato T, Tsuchiya H. Pedicle versus free frozen autograft for reconstruction in malignant bone and soft tissue tumors of the lower extremities. J Orthop Sci. 2014 Jan;19(1):156-63.
18. Deijkers RL, Bloem RM, Hogendoorn PC, Verlaan JJ, Kroon HM, TaminiauAH.Hemicortical allograft reconstruction after resection of low-grade malignant bone tumours. J Bone Joint Surg Br. 2002 Sep;84(7):1009-14.
19. Agarwal M, Puri A, Anchan C, Shah M, Jambhekar N. Hemicortical excision for low-grade selected surface sarcomas of bone. Clin OrthopRelat Res. 2007 Jun;459:161-6.
20. Agarwal M, Puri A, Anchan C, Shah M, Jambhekar N. Rotationplasty for bone tumors: is there still a role? Clin OrthopRelat Res. 2007 Jun;459:76-81.
21. Gradl G, Postl LK, Lenze U, Stolberg-Stolberg J, Pohlig F, Rechl H, Schmitt-Sody M, von Eisenhart-Rothe R, Kirchhoff C. Long-term functional outcome and quality of life following rotationplasty for treatment of malignant tumors. BMC MusculoskeletDisord. 2015 Sep 24;16:262.
22. Demiralp B, Ege T, Kose O, Yurttas Y, Basbozkurt M. Reconstruction of intercalary bone defects following bone tumor resection with segmental bone transport using an Ilizarov circular external fixator. J Orthop Sci. 2014 Nov;19(6):1004-11.
23. Khira YM, Badawy HA. Pedicled vascularized fibular graft with Ilizarov external fixator for reconstructing a large bone defect of the tibia after tumor resection. J OrthopTraumatol. 2013 Jun;14(2):91-100.
24. Chotel F, Nguiabanda L, Braillon P, Kohler R, Bérard J, Abelin-Genevois K. Induced membrane technique for reconstruction after bone tumor resection in children: a preliminary study. OrthopTraumatolSurg Res. 2012 May;98(3):301-8.
| How to Cite this article: Byregowda S, Puri A, Gulia A. Biological Methods of Reconstruction After Excision of Extremity Osteosarco. Journal of Bone and Soft Tissue Tumors May- Aug 2016;2(2):5-9 . |




