Emerging role of PET/CT in osteosarcoma
Volume 2 | Issue 1 | Jan-Apr 2016 | Page 19-21 |Nilendu C Purandare1, Venkatesh Rangarajan1
Nilendu C Purandare[1], Venkatesh Rangarajan[1]
Department of Nuclear Medicine and Molecular Imaging, Tata Memorial Hospital, Parel, Mumbai 400012
Address of Correspondence
Dr.Nilendu C Purandare
Department of Nuclear Medicine and Molecular Imaging, Tata Memorial Hospital, Parel, Mumbai 400012
Email: nilpurandare@gmail.com
Abstract
The role of PET/CT in malignant tumors has grown exponentially in the past few years. Newer methods have been investigated and older methods have been refined. This article is a brief review of its current and potential utility in osteosarcoma.
Keywords: PET/CT radioisotope scans, osteosarcoma.
Introduction:
FDG PET/CT has been used to evaluate various malignancies including those of the musculoskeletal system. It is an imaging technique that provides information about the metabolic changes associated with cancer. PET imaging uses molecules that are labelled with radio nuclides. In clinical PET practice the principal radio-isotope used is the positron emitting 18 F-FDG which is a glucose analogue labelled with radionuclide 18 F. FDG is injected intravenously and is transported from the plasma to the cells by glucose transporters (GLUT 1 & GLUT 4). It then undergoes phosphorylation within the cell by the enzyme hexokinase and is converted to FDG-6-phosphate. FDG-6-phosphate does not get further metabolised and gets trapped in the cell. Cancer cells demonstrate increased anaerobic glycolysis (Warburg effect) which is believed to be due to upregulation of glucose transporters and hexokinase and reduced levels of glucose-6-phosphatase thus limiting further metabolism of the tracer in cancer cells [1]. Thus FDG PET provides unique functional information by taking advantage of the propensity of malignant tumors to demonstrate increased glucose utilization (metabolism) as compared with normal tissue. A semiquantitative measure of the metabolic activity (corrected for the amount of radiotracer injected per kilogram of body weight and for blood glucose level) is known as the standard uptake value (SUV) . Although the degree to which both benign and malignant tumors accumulate FDG can be quite variable, malignant tumors tend to have higher SUV values. In general significant difference is noted in the SUV of benign and malignant primary bone tumors. It should be noted that the interpretation of absolute SUV values can be misleading. No cutoff value of SUV can be established in clinical practice to reliably differentiate benign from malignant primary bone tumors. Osteosarcomas (OGS) and Ewings sarcomas tend to be FDG avid whereas chondrosarcomas and soft tissue sarcomas showing a wide range of uptake depending on the histology and grade of the tumor. Since OGS is FDG concentrating tumor, FDG PET can be used for staging and restaging. FDG PET can also be used to monitor response to neo-adjuvant chemotherapy and as a prognostic and predictive marker.
Use of FDG PET in staging:
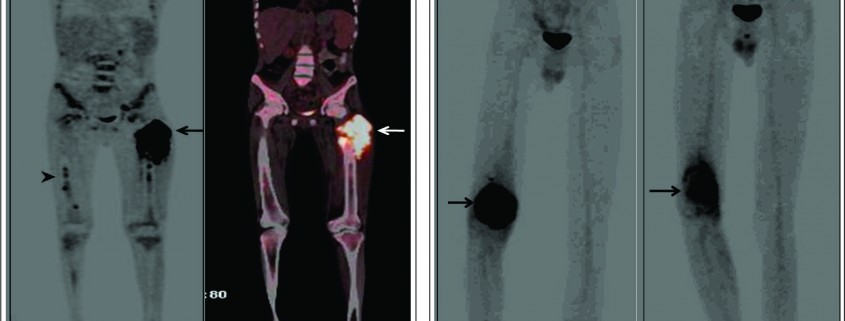
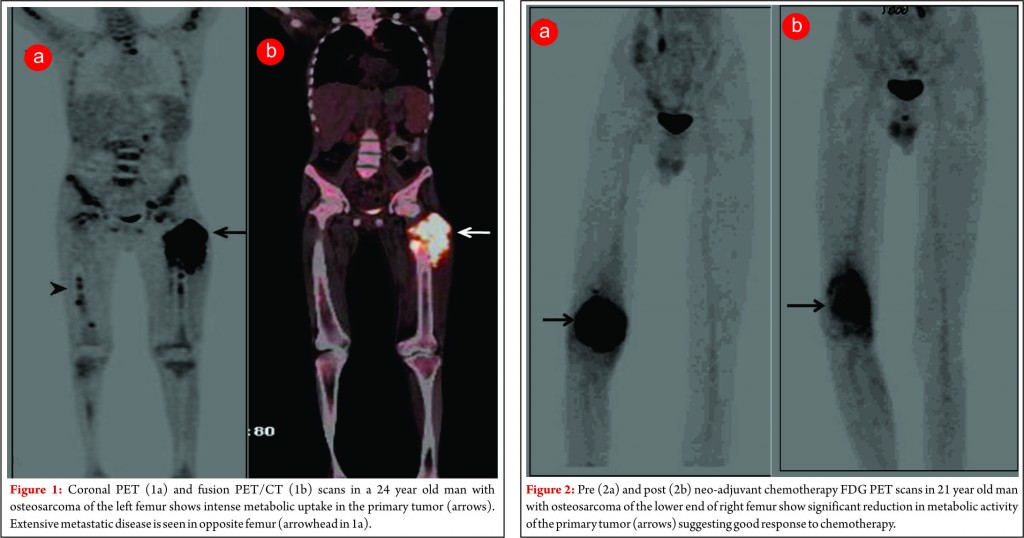
Effective treatment stratification in patients with musculoskeletal malignancies requires accurate assessment of the extent of primary tumor and evaluation for the presence of metastatic disease. MRI is the modality of choice in assessing the local extent of the primary lesion for surgical planning and PET plays a limited role. However PET may allow the noninvasive estimation of the histologic grade of tumors. The SUV of bone and soft tissue sarcomas has been used as a prognostic marker to predict patient outcomes. It has been shown that the baseline SUVmax is an independent predictor of overall survival [2]. Also FDG accumulation within a large heterogeneous tumor allows identification of the areas with the highest biologic activity. This can allow targeted biopsy from the most metabolically viable portion of the tumor, which can help in ascertaining the accurate histological grade [3]. Since OGS metastasizes to multiple skeletal sites an accurate whole body bone imaging modality is important for accurate staging. Bone scanning using 99m Tc- methylene diphosphonate(MDP) has been for several years the work horse for detection of skeletal metastases in several cancers including OGS. MDP bone scan seems to be well suited to detect skeletal involvement because of the osteoblastic nature of skeletal metastases in OGS which can be detected by a conventional MDP bone scintigraphy with a high degree of sensitivity. However MDP bone scanning can suffer from limited spatial resolution due to planar imaging. In addition the high tracer uptake in the region of the growth plates can also conceal metastatic lesions. Another limitation of bone scanning is the high number of false positives and indeterminate findings which need further confirmation with anatomical imaging. Recent studies have shown a better sensitivity of FDG PET/CT than MDP bone scan primarily due to its greater ability to detect metastases in the region of the growth plate which are often masked on bone scans [4]. Addition of CT information in an integrated PET/CT scan also reduce the number of false positives leading to better accuracy compared to bone scintigraphy. Volker et al in their study in paediatric sarcomas (Ewings, OGS & RMS) showed a higher sensitivity of FDG PET (88%) over conventional imaging (37%) for skeletal metastases from Ewings sarcoma [5]. The sensitivity however was not much different (90% for PET Vs 81% for conventional imaging) for skeletal metastases in OGS. PET was superior to conventional imaging in the correct detection of lymph node involvement (sensitivity, 95% v 25% respectively). With the availability of integrated PET/CT machines, pulmonary metastases can be diagnosed using the CT component of the PET/CT study with same accuracy as that of a chest CT obviating the need for a separate CT examination of the chest. Thus an integrated FDG PET/CT can serve as a single stop shop modality for metastatic work up of OGS patients (figure 1). Various metabolic parameters can be obtained from the FDG PET/CT study that provide valuable prognostic information. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are indicators of tumor metabolism which are independent prognostic markers and can predict metastases and survival in OGS [6].
Role of FDG PET as a surrogate marker in assessing response to neoadjuvant therapy:
Since functional and biochemical changes in a tumor in response to therapy occur much earlier than morphologic changes, FDG PET has proven to be very useful in looking at tumor viability before the changes are evident on standard morphological imaging techniques. Various studies have found a strong correlation between the degree of tumor necrosis on histology following neo-adjucvant chemotherapy and reduction in FDG concentration in Osteosarcomas [7,8]. In patients classified as having a good response to chemotherapy there is significant reduction in tumor FDG concentration (figure 2). Thus PET using FDG can be potentially used as a non-invasive surrogate to predict response as well for prognostication [9]. A multiparameter analysis technique based on kinetic 18F-FDG data (dynamic PET) of a baseline study and after 2 cycles is helpful for the very early prediction of chemosensitivity in patients with soft-tissue sarcomas receiving neoadjuvant chemotherapy. This can have potential implications on management in the form of whether to change or intensify chemotherapy or to decide whether to salvage or ampute the limb in chemo non-responsive tumors.
Role of FDG PET in detection of disease recurrence:
Conventional imaging modalities such as CT and MR imaging are limited in their ability to differentiate treatment related changes from recurrent tumor. Distortion of normal anatomy, symmetry and tissue planes following surgery or radiation therapy makes detection of tumor recurrence difficult. Also degradation of image quality due to artifiacts produced by metallic prosthesis limits evaluation of local tumor recurrence. The whole body imaging capabilities of FDG PET allow detection of local as well as distant tumor recurrence. FDG PET/CT is useful in detecting recurrence at the primary site and is often complementary to other imaging modalities [9]. It is fairly accurate in detecting sites of distant failure as well. Its potential benefits and limitations compared to conventional imaging modalities will have be studied in larger homogenous patient groups.
18F Sodium fluoride (NaF) PET scan:
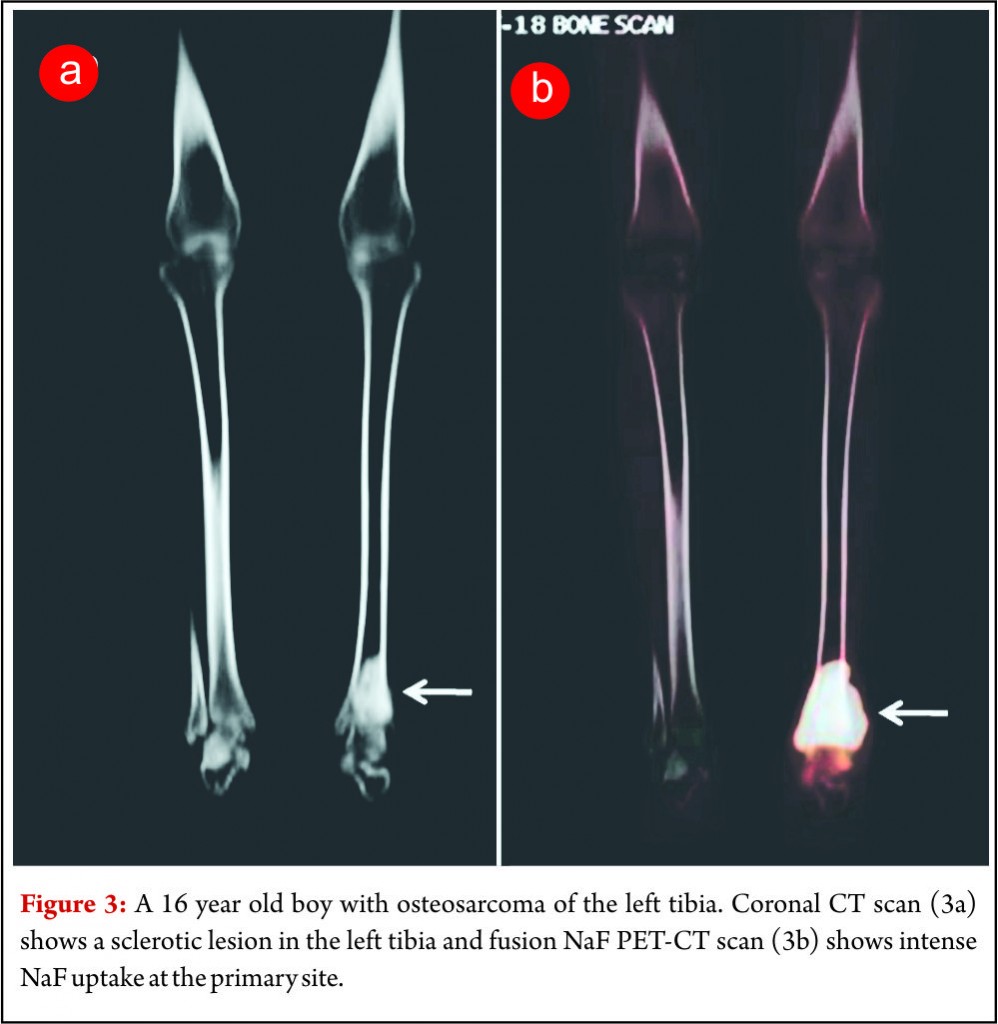
18F NaF is a bone seeking radiotracer which was introduced way back in 1962 for skeletal imaging [10]. The availability of integrated PET/CT systems has led to a renewed interest in the use of 18F-NaF for imaging skeletal metastases (Fig. 3). PET/CT imaging allows high-resolution functional imaging of the skeleton with greater sensitivity than that of planar scintigraphy/MDP bone scans. Integrated PET and CT system allows the interpretation of 18FNaF in conjunction with CT images. This enables better morphologic characterization and improved differentiation between benign and malignant lesions reducing the number if false positives and the indeterminate lesions. Studies have shown better accuracy and lesions detectability for 18F NaF PET scans as compared to MDP bone scans in several cancers.(11,12). 18F NaF PET/CT scan can image skeletal as well as lung metastases in a single examination and can be used for metastatic work up of OGS patients. Comparison of the diagnostic accuracy and cost effectiveness of FDG PET/CT and NaF PET/CT in OGS patients has not been investigated in detail and studies addressing the same need to be carried out.
References
1. Warburg O, Wind F, Negelein E. The metabolism of tumors. J Gen Physiol 1927;8:519-30.
2. Brenner W, Bohuslavizki KH, Eary JF. PET imaging of osteosarcoma. J Nucl Med 2003;44:930–942.
3. Eary JF, Conrad EU, Bruckner JD, et al. Quantitative[F-18] fluorodeoxyglucose positron emission tomography in pretreatment and grading of sarcoma. Clin Cancer Res 1998;4:1215–1220.
4. Byun BH, Kong CB, Lim I et al. Comparison of (18)F-FDG PET/CT and (99 m)Tc-MDP bone scintigraphy for detection of bone metastasis in osteosarcoma. Skeletal Radiol. 2013;42(12):1673-81.
5.Volker T, Denecke T, Steffen I et al. Positron Emission Tomography for Staging of Pediatric Sarcoma Patients: Results of a Prospective Multicenter Trial. J Clin Oncol. 2007; 25:5435-41
6.Byun BH, Kong CB, Park J, Seo Y, Lim I, Choi CW, Cho WH, Jeon DG, Koh JS, Lee
SY, Lim SM. Initial metabolic tumor volume measured by 18F-FDG PET/CT can predict
the outcome of osteosarcoma of the extremities. J Nucl Med. 2013 Oct;54:1725-32.
6.Hawkins DS, Schuetze SM, Butrynski JE, et al. [18F] Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 2005;23:8828–8834.
7.Im HJ, Kim TS, Park SY, et al. Prediction of tumour necrosis fractions using metabolic and volumetric18F-FDG PET/CT indices, after one course and at the completion of neoadjuvant chemotherapy, in children and young adults with osteosarcoma. Eur J Nucl Med Mol Imaging. 2012;39:39–49.
8. Cheon GJ, Kim MS, Lee JA, et al. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MR imaging. J Nucl Med. 2009;50:1435–1440.
9.Franzius C, Daldrup-Link HE, Wagner-Bohn A, et al. FDG-PET for detection of recurrences from malignant primary bone tumors: comparison with conventional imaging. Ann Oncol 2002;13:157–16.
10. Blau M, Nagler W, Bender MA. Fluorine-18: a new isotope for bone scanning. J Nucl Med 1962;3:332–334
11. Schirrmeister H, Guhlmann A, Kotzerke J, et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol 1999;17:2381–2389.
12. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med 1999;40:1623–1629.
| How to Cite this article:Purandare NC, Rangarajan V. EPurandare NC, Rangarajan V. Emerging role of PET/CT in osteosarcoma. Journal of Bone and Soft Tissue Tumors Jan-Apr 2016;2(1):19-21 . |