CAOS in Paediatric Bone Tumour Surgery
Vol 1 | Issue 2 | Sep – Dec 2015 | page:17-21 | James E. Archer[1], Philippa L. May [2], Lee M. Jeys[1,2*]
Author: James E. Archer[1], Philippa L. May[2], Lee M. Jeys[1,2*]
[1]The Royal Orthopaedic Hospital, Bristol Road South, Birmingham, B31 2AP, UK.
[2]College of Medical and Dental Sciences, University of Birmingham, Edgbaston, B15 2TT, UK
[3]Professor of Health and Life Sciences, Aston University, Aston Triangle, Birmingham, B4 7ET, UK
Address of Correspondence
Professor Lee M. Jeys, MBChB, MSc (Ortho. Engin.), FRCS (Tr. & Orth.), Professor of Health and Life Sciences, Aston University, Aston Triangle, Birmingham, B4 7ET, UK.
e-mail: lee.jeys@nhs.net
Abstract
Surgical navigation has been used by neurosurgery for a number of years as a method for accurately locating and resecting tumours within the brain. The anatomy of bony structures does not alter between image acquisition and the surgical procedure, therefore computer assisted technology lends itself well to use in orthopaedic surgery. Studies have demonstrated that this technology improves orthopaedic surgical accuracy across a wide breadth of procedures such as arthroplasty, knee ligament reconstructions and more recently, bone tumour surgery. This article aims to identify the importance of this new technology in paediatric bone tumour surgery and give an overview on its use.
Key Words: Computer assisted orthopaedic surgery, Paediatric, Oncology.
Introduction
Paediatric orthopaedic tumour surgery is one of the most challenging areas of orthopaedic oncology. Tumours can be found in a number of regions, with the pelvis being one of the most challenging. The removal of a tumour, post-operative complications and need for revision, all have a large impact on quality of life in paediatric patients. The complexity of the pelvic region and the associated nerve and organ structures mean that surgical excision of tumours must be very precise. Resection without Computer Asissted Orthopaedic Surgery (CAOS) has a high rate of local recurrence because of the difficulty of achieving a wide local excision [1]. The use of computer assisted technology in sarcoma surgery has improved surgical outcomes by reducing local recurrence, reducing revision rates and by decreasing the rate of amputations and nerve root damage [2,3].
The tumour types
Two tumour types are often seen in the pelvis in the paediatric population. Osteosarcomas are the most common primary bone tumour and they are most commonly seen in teenagers and young adults. They form approximately 20% of all primary bone tumours and approximately 8% of these will be found in the pelvis [4].
Ewing’s sarcoma is a rarer tumour, with around 65-75 cases per year in the UK. It is far more common in the paediatric population with a median age at diagnosis of 15 [5]. Pelvic Ewing’s sarcoma is seen in 26% of all patients [6].
In the UK, pelvic Ewing’s sarcoma are often treated pre-operatively with chemotherapy plus either radiotherapy or proton therapy. This makes surgery in the pelvis even more challenging and also has led to an increasing trend not to perform reconstruction due to the high risk of complications. This therefore makes protecting nerve roots and the hip joint essential to maintaining function.
While these tumours in the pelvis are rare and do not represent a large case load, they are often significantly advanced at the time of presentation. This is in part due to their rarity, but also due to the fact that localised symptoms only become apparent late in the condition as the pelvis can contain a large tumour without displaying symptoms. Also because of the young age of the patients, the impact on quality of life and functionality can be significant. This is especially true when considering the extent of surgical intervention that children often require. The length and complexity of surgery also means complications such as infection, dislocation and recurrence are high.
CAOS has been used less frequently in the lower limbs at our centre but has been used at other units extensively [7]. One case of the use of CAOS in the lower limb is presented in this article. Other applications for CAOS include its use in biopsy and for radio-frequency ablation [8].
The Challenge of Pelvic Surgery
There are a number of features which make pelvic oncological surgery challenging and it therefore requires experienced and highly skilled surgical intervention.
The first feature of note is the average size of tumours, at presentation primary pelvic tumours tend to be extremely large as the symptoms of a tumour in this region are often attributed to other causes such as musculoskeletal pain. Furthermore, swelling can be non-palpable due to its location and the overlying muscle. These features in combination tend to mean that pelvis tumours are large at presentation, on average 628cm3 [9].
The underlying anatomy of the pelvis itself also makes this a challenging area to perform surgery. The pelvis is a large, complex three-dimensional structure with numerous nerves and friable soft tissues present. Because of its size and complexity, patients often have to be moved intra-operatively to facilitate appropriate access to the pelvis.
The tumours themselves also contribute to the difficulty of surgery. They frequently contain cystic lesions which may burst, furthermore they also can be weaker than the surrounding healthy bone structure leading to fracturing of the tissue to be removed.
Furthermore, achieving a wide local excision of the tumour can be challenging. As discussed, these tumours tend to be discovered late, therefore they can have invaded into local soft tissue structures. Attempting to maintain joint, hip capsule and articular surfaces can help to maintain function but may impact on the completion of a wide local excision. These difficulties in resection of the tumour have led to the high rate of local recurrence reported [10], due to inadequate resection margins, both intra-lesional and marginal.
One study looked at the success of wide local excision margins in the pelvis, in experienced surgeons, operating on model pelvises in ideal conditions. The probability of them obtaining a suitable margin was 52%, highlighting the difficulty of obtaining appropriate margins for surgical resection [11].
The primary aim of the surgery is of course excision of the tumour but an important secondary outcome is to ensure function. Reconstruction using a one-size fits all prosthesis is not possible in the pelvis due to the complexity of the mechanics of the pelvis. Therefore, individual prosthesis are required, which fit effectively to prevent further revision being required. In those fitted with an endoprosthesis the 5 year survival of the prosthesis was 76% [10], compared with 87% survival at 12 years for those who undergo massive allograft reconstruction [12].
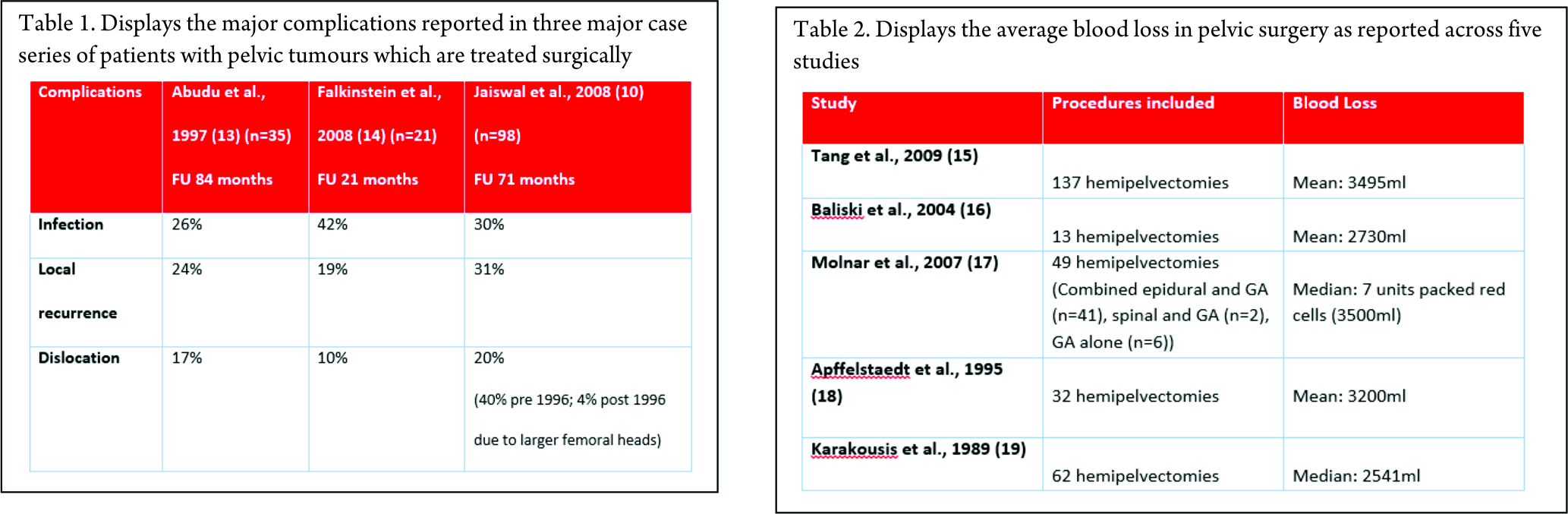
Once all of these other features have been overcome, the rates of complication are also high, due to all of the factors identified above. Three major case series have reported their complications and the data is included in the table below. They have reported complication rates of greater than 50%, with infection, local recurrence and dislocation being the major contributors as shown below (Table 1).
How can we improve these outcomes?
There are a number of features that have been targeted to try and improve outcomes.
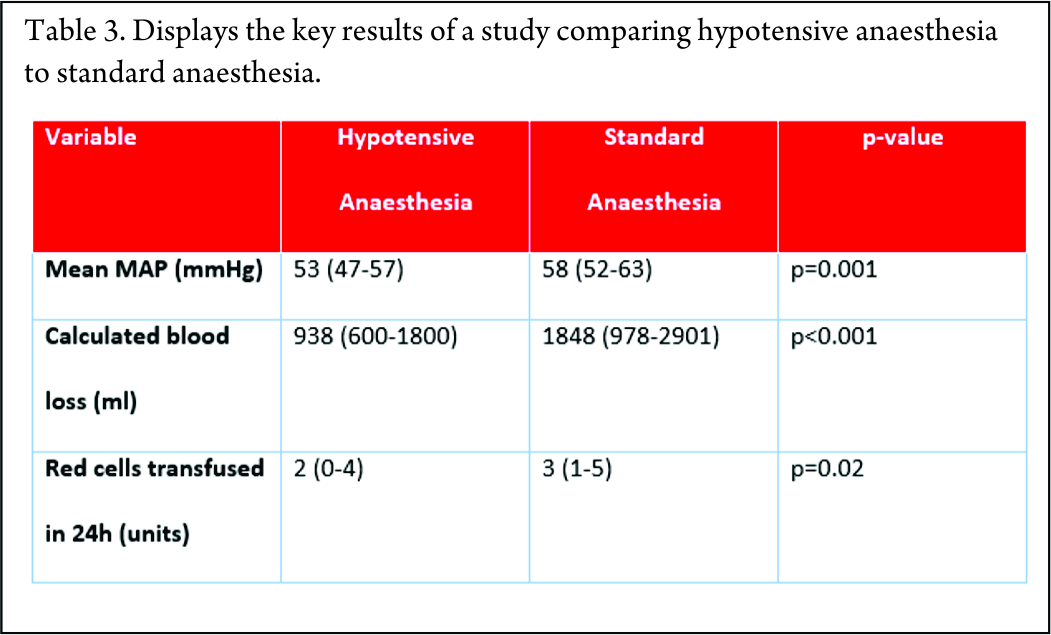
Firstly, blood loss during surgery can be significant, as shown in the studies below. Every study shows an average blood loss of greater than 2.5 litres, which in a paediatric population represents a significant volume of blood loss (Table 2).
One method for reducing blood loss is hypotensive anaesthesia. By maintaining patients at a lower mean arterial pressure, the amount of bleeding can be reduced. A retrospective study using hypotensive epidural anaesthesia compared to standard anaesthetic technique was performed at The Royal Orthopaedic Hospital, Birmingham [20]. This showed that blood loss was significantly reduced by using this technique (Table 3).
The other big advance has been the use of CAOS. CAOS works effectively in the pelvis for a number of reasons. Firstly, the bony structure of the pelvis allows for multiple bony landmarks to be used as reference points. The registration of landmarks allows the computer to orientate itself correctly within space and therefore build up a correlation between the saved images and the three dimensional structures that are present. This correlation is what allows such accuracy at the time of surgery and helps to ensure an adequate wide local excision by accurately delivering the pre-operative planned resection to within 1mm.
The image required for CAOS is also important as both CT and MRI offer benefits. CT images provide good detail of the bony structures, but MRI provides more information on the soft tissue structures and the extent of the tumours. Therefore, fusion of these imaging modalities has been performed [21] which allows for a more complete pre-operative planning image. As technology advances further imaging modalities have been fused into this process including PET scans and angiograms.
There have been some reports of improved intraoperative registration being achieved by implanting four Kirschner wires, two in the iliac crests and two in the posterosuperior iliac spines, to act as permanent markers. The implantation of these wires helps to offset any error that may be produced by orientation difficulties and further enhances the accuracy of surgery within a delicate region [22].
Use of CAOS has shown a reduction in local recurrence rates from 26% to 13% at a mean follow up of 13.1 months [2]. While this has not completely removed local recurrence, it is a significant reduction in preliminary practice. The authors suggest that as surgeons become more practiced in using the CAOS technology, further improvements could be seen. The importance of minimising registration errors is highlighted, as this can help to ensure good accuracy of the surgery and therefore good surgical margins can be achieved. One element of this registration accuracy is to ensure that the time between image acquisition and surgery is minimised.
Finally, the design of custom made implants has also been made possible by the precise planning and good quality pre-operative images. Using the planning images, and the exact measurements and angles allowed by this process, the engineer can effectively design a custom implant which will fit exactly. This has previously not been possible as the surgeon would need to make a judgement at the time of the procedure regarding the extent of resection, so a pre-planned prosthesis, custom made to fit exactly to the patient was not always feasible. Good communication between the engineer and the planning surgeon is essential to ensuring that the implants will work effectively.
Case Illustrations
To highlight the importance of CAOS we now briefly discuss two cases which were performed at The Royal Orthopaedic Hospital, Birmingham using CAOS.
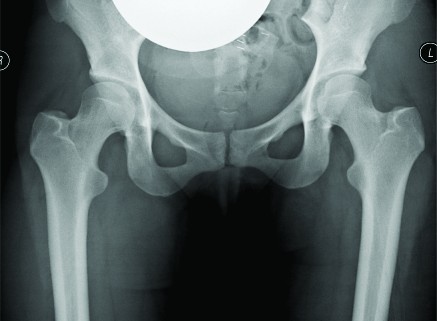
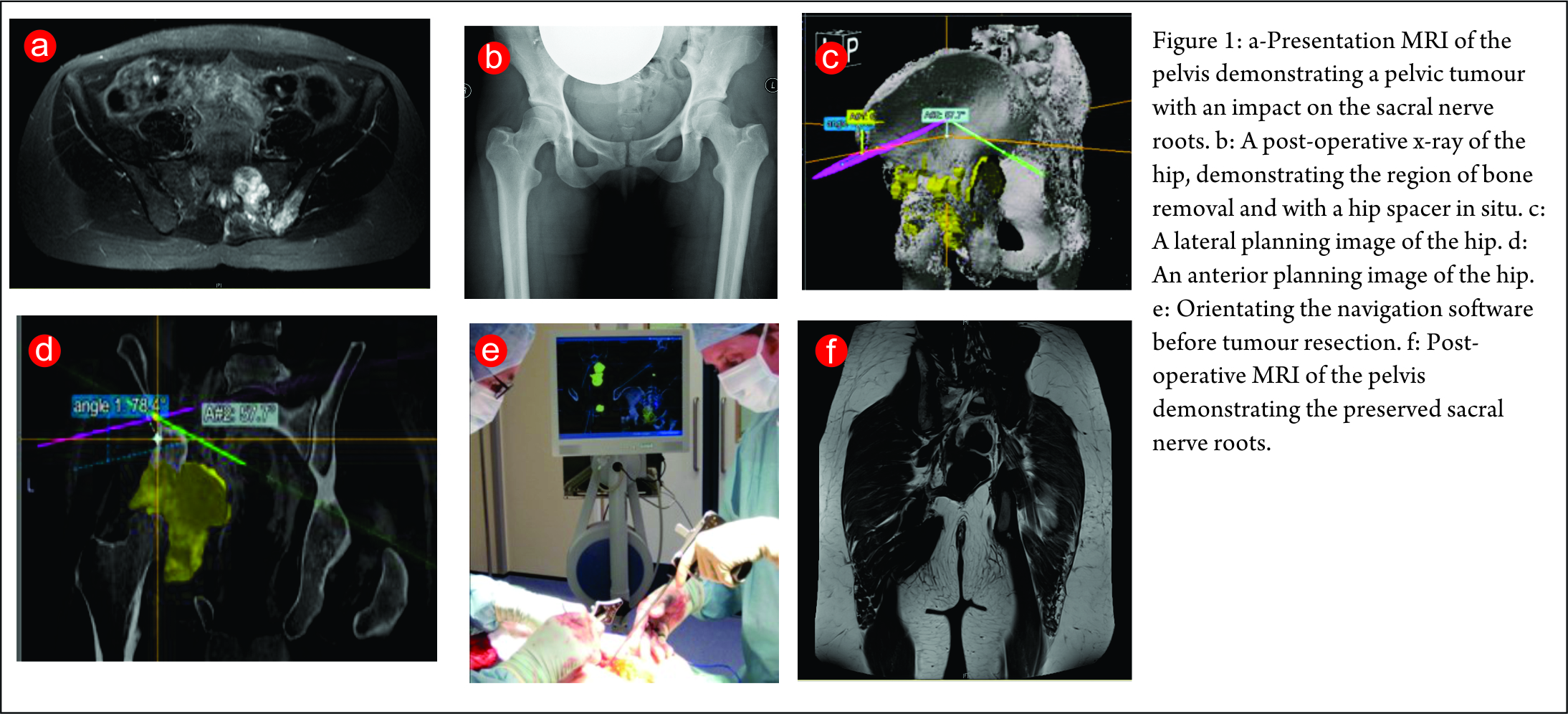
The first case is a case of a pelvic Ewing’s sarcoma. The presentation MRI is shown below (Fig. 1). The case was discussed at the National Ewing’s sarcoma MDT meeting, the location of the tumour made surgical resection very challenging as there was significant risk of damage to the L5, S1 and S2 nerve roots. She was initially given four cycles of chemotherapy and then re-discussed at the MDT meeting. Despite the high risk, the decision was made to combine surgery with radiotherapy as it was felt this would probably give the best outcome. The significant morbidity risks were discussed with the patient and her family. They decided to proceed with surgery. She underwent a computer assisted hemisacrectomy after pre-operative chemotherapy and radiotherapy with a good response. The images below show images from the planning software and then the orientation of the navigation technology (Fig. 1c,d,e). The operation was successful and managed to preserve the nerve roots and histology confirmed complete necrosis of the tumour. The images below show her post-operative recovery (Fig. 1b,f). No further radiotherapy was therefore required. She made a good post-operative recovery and progressed so well that she was able to Ski one year after the procedure, where she fell and fractured her pelvis. This has now healed and she can walk an unlimited distance and is wakeboarding!
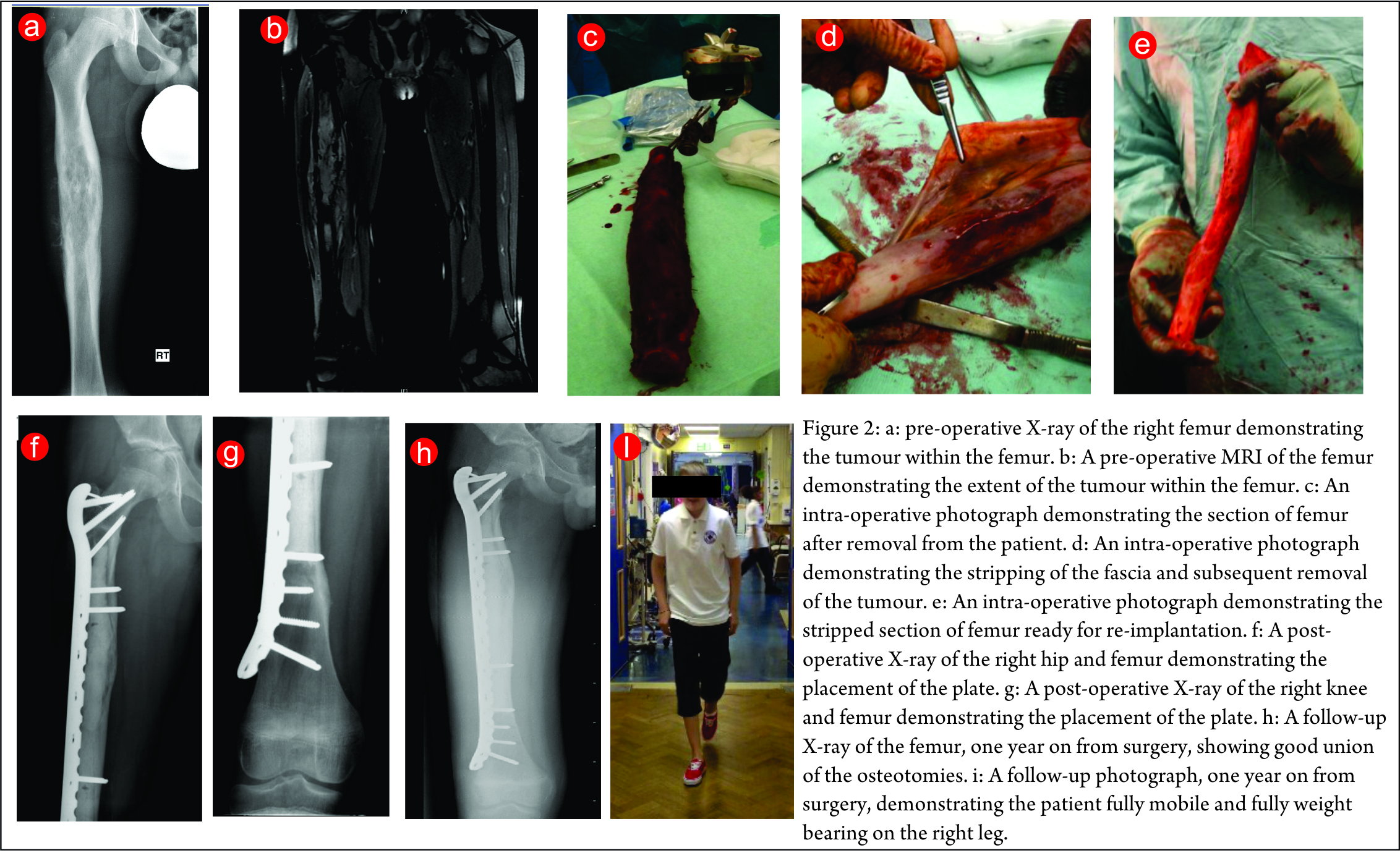
The second case is a lower limb Ewing’s sarcoma. The presentation X-ray and MRI images are shown in (Fig. 2a,b). He underwent neo-adjuvant chemotherapy and had a good response to this. He then underwent a computer assisted radiation reimplantation of his right femur. His femur was removed and irradiated with 90 Gy of radiotherapy (Fig. 2 c,d,e). He made a good post-operative recovery and histology confirmed complete necrosis of his tumour. The post-operative images below show good fixation was achieved (Fig. 2 f,g). He completed his post-operative chemotherapy and made an excellent recovery with full weight bearing and good union of his osteotomies (Fig. 2h,i) show his follow-up x-ray and an image of him walking to demonstrate normal function.
Conclusion
The use of CAOS has increasing evidence of providing improved outcomes. Early results seems to suggest decreased local recurrence, reduced rates of revision and decreased need for amputation (2). One of the major improvements offered by CAOS is the reduction in intra-lesional resection rates (1,23,24).The increased accuracy afforded by CAOS also allows for better fitting implants with better biomechanics, therefore helping to reduce complications and the rate of revision.
Surgical planning time is currently longer when CAOS is used, however it is felt that as surgeons become more practiced this time will reduce. Furthermore, the currently increased time taken is likely worth it for the improvement in outcomes. Currently unpublished data from this centre also shows that there is a reduced operative time when CAOS is used.
While cost-effectivity of this approach has not been assessed, the reduced complications and potential for reducing local recurrence would appear to point to a long-term cost saving by using this approach to paediatric bone tumour surgery in the pelvis.
References
1. Ozaki T, Flege S, Kevric M, Lindner N, Maas R, Delling G, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol Off J Am Soc Clin Oncol. 2003 Jan 15;21(2):334–41.
2. Jeys L, Matharu GS, Nandra RS, Grimer RJ. Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Jt J. 2013 Oct;95-B(10):1417–24.
3. Wong KC, Kumta SM. Computer-assisted tumor surgery in malignant bone tumors. Clin Orthop. 2013 Mar;471(3):750–61.
4. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152.
5. Grimer R, Athanasou N, Gerrand C, Judson I, Lewis I, Morland B, et al. UK Guidelines for the Management of Bone Sarcomas. Sarcoma. 2010;2010:1–14.
6. Bone Cancer Research Trust. Ewing’s sarcoma information, Version 2 [Internet]. 2013. Available from: http://www.bcrt.org.uk/bci_what_is_ewings_sarcoma.php
7. Aponte-Tinao L, Ritacco LE, Ayerza MA, Muscolo DL, Albergo JI, Farfalli GL. Does intraoperative navigation assistance improve bone tumor resection and allograft reconstruction results? Clin Orthop. 2015 Mar;473(3).
8. Gerbers JG, Stevens M, Ploegmakers JJ, Bulstra SK, Jutte PC. Computer-assisted surgery in orthopedic oncology: Technique, indications, and a descriptive study of 130 cases. Acta Orthop. 2014 Dec;85(6):663–9.
9. Jeys et al. Computer navigation assisted surgery for pelvic and sacral tumours: experience of a tertiary centre. In Gothenburg, Sweeden; 2013. p. 08.104.
10. Jaiswal PK, Aston WJS, Grimer RJ, Abudu A, Carter S, Blunn G, et al. Peri-acetabular resection and endoprosthetic reconstruction for tumours of the acetabulum. J Bone Joint Surg Br. 2008 Sep;90(9):1222–7.
11. Cartiaux O, Docquier P-L, Paul L, Francq BG, Cornu OH, Delloye C, et al. Surgical inaccuracy of tumor resection and reconstruction within the pelvis: an experimental study. Acta Orthop. 2008 Oct;79(5).
12. Campanacci D, Chacon S, Mondanelli N, Beltrami G, Scoccianti G, Caff G, et al. Pelvic massive allograft reconstruction after bone tumour resection. Int Orthop. 2012 Dec;36(12):2529–36.
13. Abudu A, Grimer RJ, Cannon SR, Carter SR, Sneath RS. Reconstruction of the hemipelvis after the excision of malignant tumours. Complications and functional outcome of prostheses. J Bone Joint Surg Br. 1997 Sep;79(5):773–9.
14. Falkinstein Y, Ahlmann ER, Menendez LR. Reconstruction of type II pelvic resection with a new peri-acetabular reconstruction endoprosthesis. J Bone Joint Surg Br. 2008 Mar;90(3):371–6.
15. Tang X, Guo W, Yang R, Tang S, Ji T. Evaluation of blood loss during limb salvage surgery for pelvic tumours. Int Orthop. 2009 Jun;33(3):751–6.
16. Baliski CR, Schachar NS, McKinnon JG, Stuart GC, Temple WJ. Hemipelvectomy: a changing perspective for a rare procedure. Can J Surg J Can Chir. 2004 Apr;47(2).
17. Molnar R, Emery G, Choong PFM. Anaesthesia for hemipelvectomy–a series of 49 cases. Anaesth Intensive Care. 2007 Aug;35(4):536–43.
18. Apffelstaedt JP, Driscoll DL, Karakousis CP. Partial and complete internal hemipelvectomy: complications and long-term follow-up. J Am Coll Surg. 1995 Jul;181(1):43–8.
19. Karakousis CP, Emrich LJ, Driscoll DL. Variants of hemipelvectomy and their complications. Am J Surg. 1989 Nov;158(5):404–8.
20. Freeman A., Thorne C., Gaston L., Shellard R., Jeys L. The effect of hypotensive epidural anaesthesia (HEA) on blood loss during pelvic and sacral tumor surgery. In Athens, Greece; 2015. p. FC – 018.
21. Wong KC, Kumta SM, Antonio GE, Tse LF. Image fusion for computer-assisted bone tumor surgery. Clin Orthop. 2008 Oct;466(10):2533–41.
22. Cho HS, Kang HG, Kim H-S, Han I. Computer-assisted sacral tumor resection. A case report. J Bone Joint Surg Am. 2008 Jul;90(7):1561–6.
23. Jeys L, Grimer R, Carter S, Tillman R, Abudu S. OUTCOMES OF PRIMARY BONE TUMOURS OF THE PELVIS – THE ROH EXPERIENCE. J Bone Joint Surg Br. 2012 Apr 1;94-B(SUPP XIV):39–39.
24. Fuchs B, Hoekzema N, Larson DR, Inwards CY, Sim FH. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin Orthop. 2009 Feb;467(2):510–8.
| How to Cite this article: Archer JE, May PL, Jeys LM. CAOS in Paediatric Bone Tumour Surgery. Journal of Bone and Soft Tissue Tumors Sep-Dec 2015;1(2):17-21. |

Dr. Philippa L. May |