Evaluation of Osteogenic Sarcoma
Volume 2 | Issue 1 | Jan-Apr 2016 | Page 8-12 | Mandip Shah, Chetan Anchan.
Authors: Mandip Shah[1], Chetan Anchan[2]
[1] SPARSH orthopedic Oncology Clinic, 9th floor, Medicare building, Ellisbridge, Ahmedabad. India
[2]Bombay Hospital & Medical Research Centre, Mumbai, India
Address of Correspondence
Dr. Mandip C Shah (M.S Ortho)
SPARSH orthopedic Oncology Clinic
9th floor, Medicare building, Ellisbridge, Ahmedabad, 380006
Email: drmandip@gmail.com
Abstract
Primary malignant bone tumors are very rare diseases and the initial symptoms and signs can be vague and nonspecific resulting in such patients receiving, at best, some symptomatic care, with the expectation that the problem would resolve naturally. Often the patient may wait out a period of weeks or months hoping that the problem would settle on its own with some home remedy. The foundation of optimal outcome in the treatment of any malignant disease is early detection and correct diagnosis. Osteosarcoma (also called as osteogenic sarcoma) is a high grade malignant disease which is fatal unless treated in time. Early detection and correct diagnosis can make a big difference in the outcome of treatment of these diseases. Awareness of these conditions and the knowledge of vulnerable age groups is the perfect start for achieving this goal. A detailed history of the presenting complaints and a thorough clinical evaluation of the patient will provide vital clues that should alert the clinician to a possible bone tumor. Radiographs and MRI form the mainstay of radiological investigation of bone tumors. Besides aiding in detection of the bone tumor, radiographs are of vital diagnostic value; whereas MRI provides very detailed anatomical information of the extent of the disease. No diagnosis of a malignant bone tumor is complete without histological confirmation of the disease and therefore biopsy is the final step in the diagnostic evaluation of a suspected malignant bone tumor. As for all malignant tumors, staging investigations must be done before starting treatment for osteosarcoma.
Keywords: Osteogenic Sarcoma, diagnosis, evaluation.
Introduction
Osteosarcoma is a malignant tumor of mesodermal origin where the tumor cells produce bone or osteoid [1]. It is the most common primary malignant bone tumor, excluding hematopoietic bone tumors [1, 2]. Despite the simple and clear definition of this disease, the term osteosarcoma represents a family of tumors with significant diversity in its histological features, grade and clinical behavior [1]. However, it is a very rare disease and represents less than 1% of all cancers diagnosed in the United States [4]. It is seen most frequently in children and adolescents peaking in the second decade, which coincides with the growth spurt [3]. In these young patients, it chiefly affects the metaphysis of long bones. The most commonly involved region is the knee with the distal femur being the most affected, followed by the proximal tibia [3]. Besides the appendicular skeleton, osteosarcoma can affect other bones too; including the skull, axial, and very rarely, the acral bones. Although the majority of osteosarcomas occur in children and adolescents, there is a second spike in its incidence which is seen in the elderly – above the age of 60 years [5]. Unlike in the younger patients where most of the osteosarcomas arise de novo, a large number of osteosarcomas in the elderly arise in preexisting bone pathologies like Paget’s disease, fibrous dysplasia and in areas previously treated with radiation for some other cause [6, 7]. Males are more frequently affected than females. The overall world male to female ratio of osteosarcoma, in the age group of 0-24 years is 1.43:1 [8]. This difference steadily decreases with increasing age [8]. Osteosarcoma is a high grade malignant tumor which is fatal unless detected and diagnosed in time, and treated appropriately. Due to the rarity of this disease and lack of very obvious early clinical diagnostic features, there is often a delay in its detection and diagnosis; adversely affecting the outcome of treatment. Early detection and correct diagnosis gives the patient the best start to a long and difficult fight. In this article we describe a simple, logical and practical approach to evaluating a patient for a suspected bone tumor.
Evaluation of Osteosarcoma
A systematic approach is involved in the evaluation of any suspected bone neoplasm so as to reach a correct diagnosis, following which optimal treatment can be planned. As for most bone tumors, in cases of suspected osteosarcoma, this involves detailed clinical, radiological and histological evaluation.
Clinical evaluation
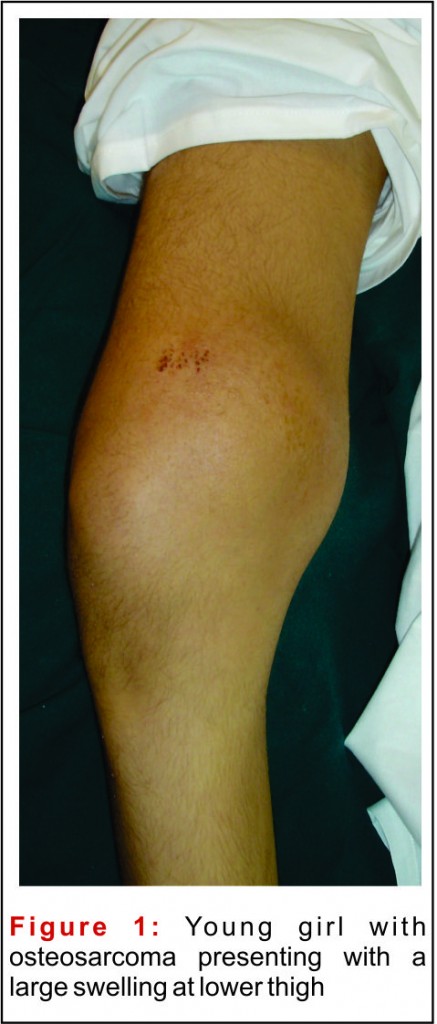
The three chief presenting symptoms of any bone tumor are pain, swelling and disability (Fig 1). Of these, pain is the most common presenting complaint in osteosarcoma, which, to begin with, may be experienced during activity that loads the affected bone. The pain may be in the form of a dull ache or such non-specific nature which could be attributed to more common causes like bone/muscle/ligament injury, articular pathologies etc. The duration of this pain may range from days to months. Special attention must be paid to patients in the vulnerable age group, especially when the complaint is unilateral, localized, persistent or progressive. Some individuals may associate the onset of the disease with some past injury. However, there is no evidence to substantiate that injury can lead to genesis of osteosarcoma.
Unexplained musculoskeletal pain should be taken very seriously, especially in children and adolescents, and should not be dismissed without proper investigation. In general, one must rule out a neoplastic cause for the musculoskeletal pain if one or more of the points mentioned below are noted.
1) Unilateral and localized extremity pain without a known cause
2) Pain intensity/duration/evolution in conflict with assumed routine cause
3) Pain with swelling
4) Pain since weeks/months
5) Persistent or progressively increasing pain
6) Pain, only temporarily / not relieved – with conservative care (rest and analgesics)
7) Pain causing disability, or affecting activity which is considered normal for the patient
8) Pain aggravated/triggered with activity
9) Rest/night pain
The next common presenting complaint is swelling in the affected region. This swelling may be visible or/and palpable – depending on the size and location of the tumor. It is unusual for a patient of osteosarcoma to present with a painless swelling, with the possible exception of parosteal osteosarcoma. Unlike pain, which is far more likely to be due to some injury or many such routine causes, a swelling is clearly an indication of a pathology, the significance of which should be investigated without further delay. Again, one must be aware that there are many causes of bony swelling ranging from infection to various types of benign and malignant tumors, and tumor like conditions. It is useful to get answers to the following questions when a patient presents with a bony swelling.
1) Location and size of swelling?
2) Is the swelling painful or painless?
3) Did the pain lead to discovery of the swelling or an existing swelling became painful?
4) Duration – Days/weeks/months/years?
5) Rate of growth?
6) Solitary or multiple?
Pain or/and swelling may result in some form of disability. Pain in the lower limb may affect ambulation or cause limitation of range or function across the adjacent joint. Rarely, patients with osteosarcoma may present with a pathological fracture. Pathological fracture is uncommon in osteosarcoma as majority of these patients would have sought medical attention before such an event occurred [9]. The risk of pathological fracture is higher in telangiectatic variant of osteosarcoma as it is a lytic expansile disease. Pathological fracture in children and adolescents is far more likely to be due to benign conditions like simple bone cyst, fibrous dysplasia, aneurysmal bone cyst, etc. Nevertheless, an occasional telangiectatic osteosarcoma can present in a similar way. Therefore, it becomes essential that a clear diagnosis of the cause of the fracture is established before deciding on the treatment. To identify a pathological fracture, one must rely a lot on the circumstances of the fracture rather than the X-ray alone. One must seek answers to the following questions:
1) How did the fracture occur? Was the cause significant or trivial?
2) Did the patient have complaints of pain/swelling/disability in the affected region prior to the fracture?
3) Has the patient suffered similar fractures in the past in the same location or in other bones?
There are generally no systemic or constitutional symptoms due to osteosarcoma, unless the disease is very advanced with extensive metastases. Lungs are the most common site for metastasis and these patients mainly present with breathlessness [10]. Some patients may present with bone metastases, which is the most common site for extra-pulmonary metastasis [10]. Regional nodal metastases and systemic metastasis to other organs/tissue is rare [10].
Clinical Evaluation
A detailed clinical examination is the next step in the evaluation of a patient with suspected bone tumor. A detailed local examination assessing the exact location, size and extent of the lesion should be done. The findings could range from subtle signs like raised local temperature/deep tenderness/vague swelling, to a very obvious painful, tender and large bony swelling with stretched hypervascular overlying skin and restriction of associated joint function. One must also make a note of the function of the adjacent joint and any distal neuro-vascular deficit. Although nodal metastasis is very rare in osteosarcoma, as a routine practice, regional draining nodes should be examined.
Blood investigations
There are no specific serum markers for osteosarcoma. Patients with high pre-treatment Lactate Dehydrogenase (LDH) levels have been reported to have 20% lower disease free survival as compared to those with normal LDH levels [12]. Similarly, a high pretreatment level of serum Alkaline Phosphatase has been reported to be an independent adverse prognostic marker in the outcome of treatment of non-metastatic osteosarcoma of extremities [13].
Radiological Evaluation
The next logical step in the work-up of a suspected bone tumor is imaging. MRI and CT scan have revolutionized medical imaging of human body and have contributed hugely to the success in the treatment of musculoskeletal tumors. However, when it comes to diagnosing bone tumors, the imaging modality that matters the most is the plain radiograph. With few exceptions, all other imaging modalities help mainly in understanding the anatomical extent of the disease and are of limited/selective diagnostic value.
Radiograph
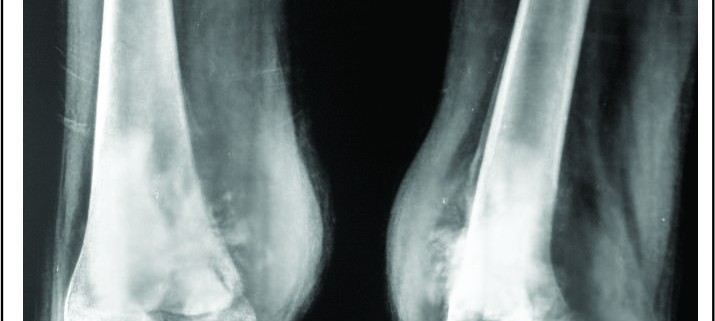
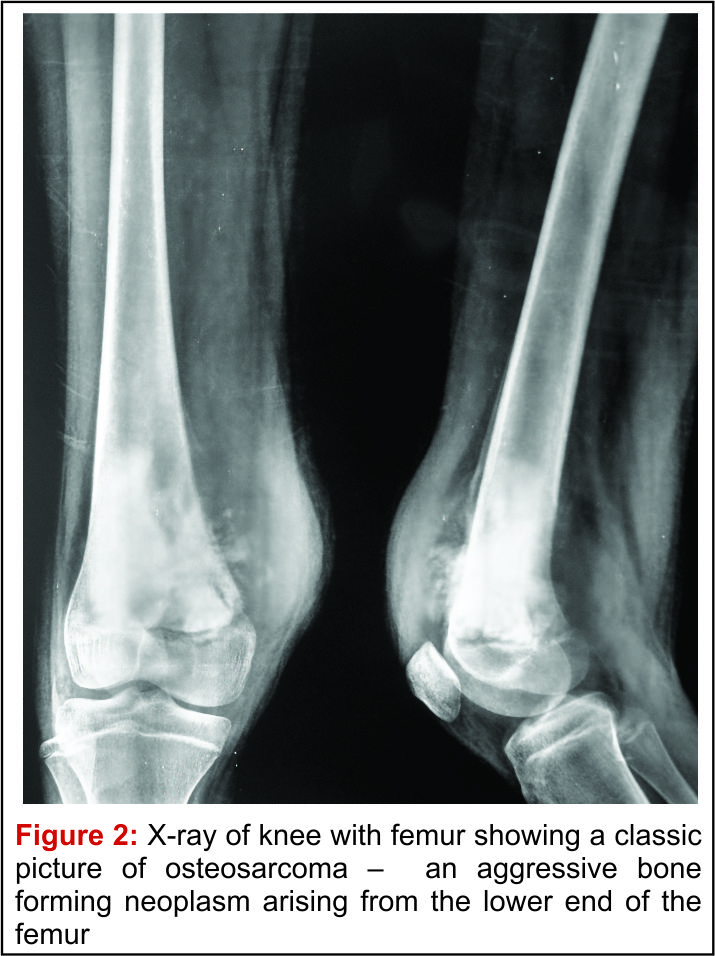
A good quality plain radiograph in two perpendicular planes screening the entire bone should be taken. Conventional osteosarcoma can have varying appearance on the plain X-ray. It appears like an ill-defined cloudy/fluffy radiodensity in the bone which may show a mixture of lytic and sclerotic areas. The borders of this lesion are ill-defined and it appears to permeate through the normal bone around. It does not have a precisely identifiable border on the X-ray and there is a wide zone where the disease merges with the normal bone. This is described as a “wide zone of transition” and is a sign of an aggressive disease. Once the disease breaches the cortex, it lifts up the periosteum which elicits a periosteal reaction which may have varying appearances described as a sunburst /spiculated/lamellated reaction or as a Codman triangle. All such patterns of periosteal reaction, which is described as an interrupted periosteal reaction, are a very important sign of a potentially malignant disease. Large osteosarcomas can have soft tissue extension of the disease which appears as a soft tissue shadow on the X-ray and which may show cloudy/fluffy radiodensities within it. Besides these classic X-ray findings of a conventional osteosarcoma, many of the rare variants of osteosarcoma have X-ray characteristics which are unique to that particular sub-type and could help in suspecting/identifying them [11] (Fig 2).
MRI
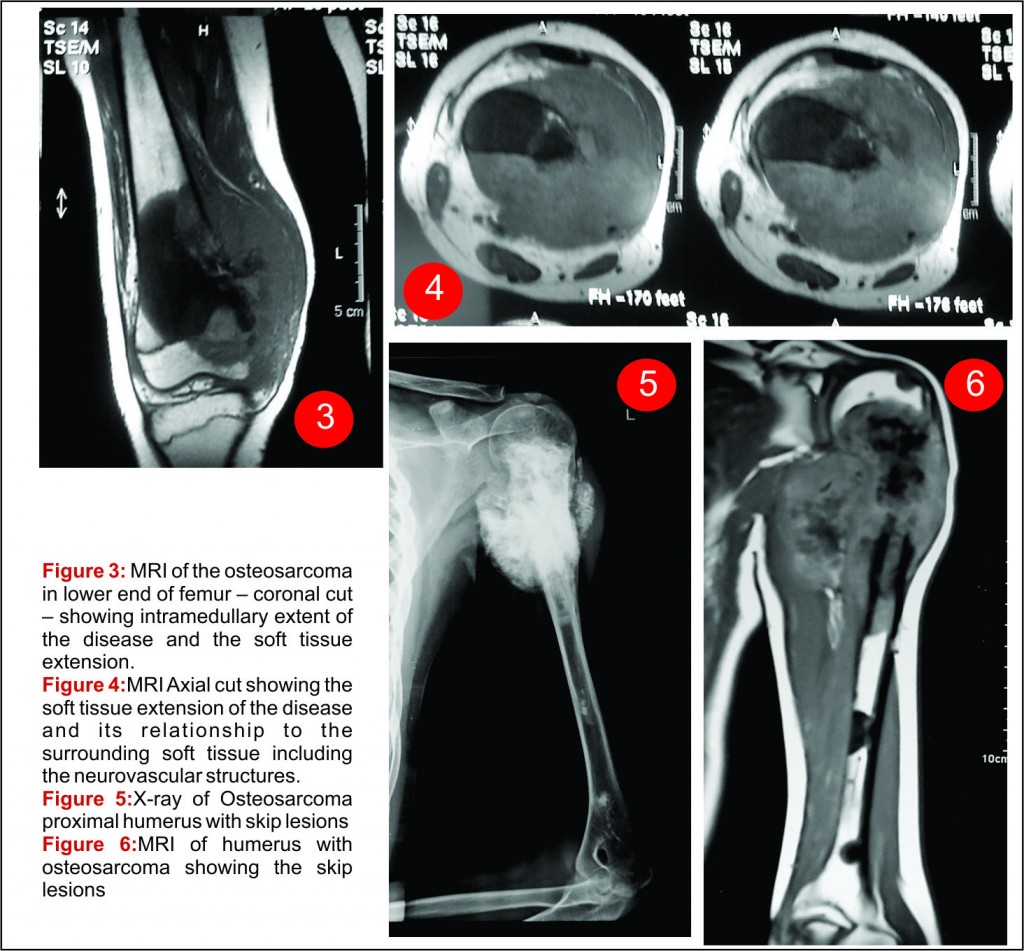
MRI is the investigation of choice in suspected case of osteosarcoma for local staging [14, 15]. One must insist on a contrast study screening of whole involved bone to rule out any skip lesion [16]. MRI must ideally be done before the biopsy as it helps in planning the biopsy approach and also in targeting representative areas within the lesion, avoiding areas of tumor necrosis. Also, doing an invasive procedure before the MRI may alter the MRI findings by causing procedure related artifacts and edema. MRI gives useful information on intra medullary and extramedullary extent of disease, presence of any skip lesion, proximity of the tumor to the neurovascular structures and involvement of joint / physeal plate etc (Fig. 3,4,5). An additional MRI study is usually advised after the completion of neoadjuvant chemotherapy, just prior to the surgery for local management of the osteosarcoma, Post chemotherapy response prediction can be assisted with MRI as well. Reduction in the size of the soft tissue mass/vascularity/reactive zone and intramedullary edema, thickening of the peritumoral capsule and presence of necrosis are some of the signs of good response to chemotherapy. Assessment of chemotherapy response is best done by contrast enhanced, diffusion weighted study [17,18].
Histopathological Evaluation
Although, the diagnosis of osteosarcoma can be assumed with a fair degree of certainty based on the clinical and radiological findings, under no circumstances the treatment can be started without histological confirmation. Osteomyelitis, osteoblastoma, bone metastasis, lymphoma, GCT, ABC, are the radiological differentials to osteosarcoma. On the other hand, one cannot rely only on biopsy alone for diagnosis of osteosarcoma – the classic example is of callus which can be indistinguishable from osteosarcoma on histology. Hence it is very important to correlate clinical, radiological and histological information to reach a diagnosis of any bone tumor. Biopsy is a procedure where a representative sample of the disease tissue is procured for histological studies. There are many ways this sample can be obtained. The routine procedures are open biopsy, needle biopsy and fine needle aspiration cytology (FNAC). Before doing a biopsy, it is advisable to complete all the radiological imaging studies. The most important step in planning a biopsy of any bone tumor is to decide on the approach. This is very important because, during the definitive surgery of a malignant bone tumor, the entire biopsy tract including the skin scar is excised en masse with the tumor. Therefore, it is very essential that the biopsy incision is placed in the line of the incision of the future surgery [19]. Open biopsy is a surgical procedure where tissue samples are obtained through a minor surgical procedure. The incision should be just adequate to obtain the deeper tissue and should be parallel to the long axis of the limb, in a location that would allow its easy excision along with the tumor at the time of definitive surgery. Needle biopsy is a procedure where tissue samples are obtained using a bone biopsy needle through a small stab incision. There are several advantages of needle biopsy over open biopsy. It causes limited contamination of the biopsy tract as it has a small footprint, which makes excision of the biopsy tract much easier during definitive surgery and also results in much less loss of skin as a result of the same. Besides this, it has several advantages like faster recovery, less hospital stay, lower cost etc. Also, the longer reach of the needle makes it easier to sample different regions of the tumor. As with open biopsy, the placement of the biopsy incision is important. Also, sampling of different regions of the lesion should be done through the same incision by just changing the angle of the needle and not through another skin incision. The only relative disadvantage of this procedure as compared to open biopsy is perhaps the smaller quantity of tissue sample that may be obtained, which could prove challenging to the pathologist to work on. However, in experienced hands this is generally not a problem. Frozen section may be used to confirm that the tissue sample obtained is representative. However, it should not be relied on to make a definitive diagnosis of bone tumors. FNAC as a procedure has many advantages, being minimally invasive and practically without morbidity, and with the least risk of tumor seeding along the biopsy tract. There are many reports of bone tumor diagnosis using FNAC. However, it has some limitations especially related to adequate representative tissue sampling and hence is not ideal for a definitive diagnosis of bone tumors like osteosarcoma [20].
Staging in Osteosarcoma
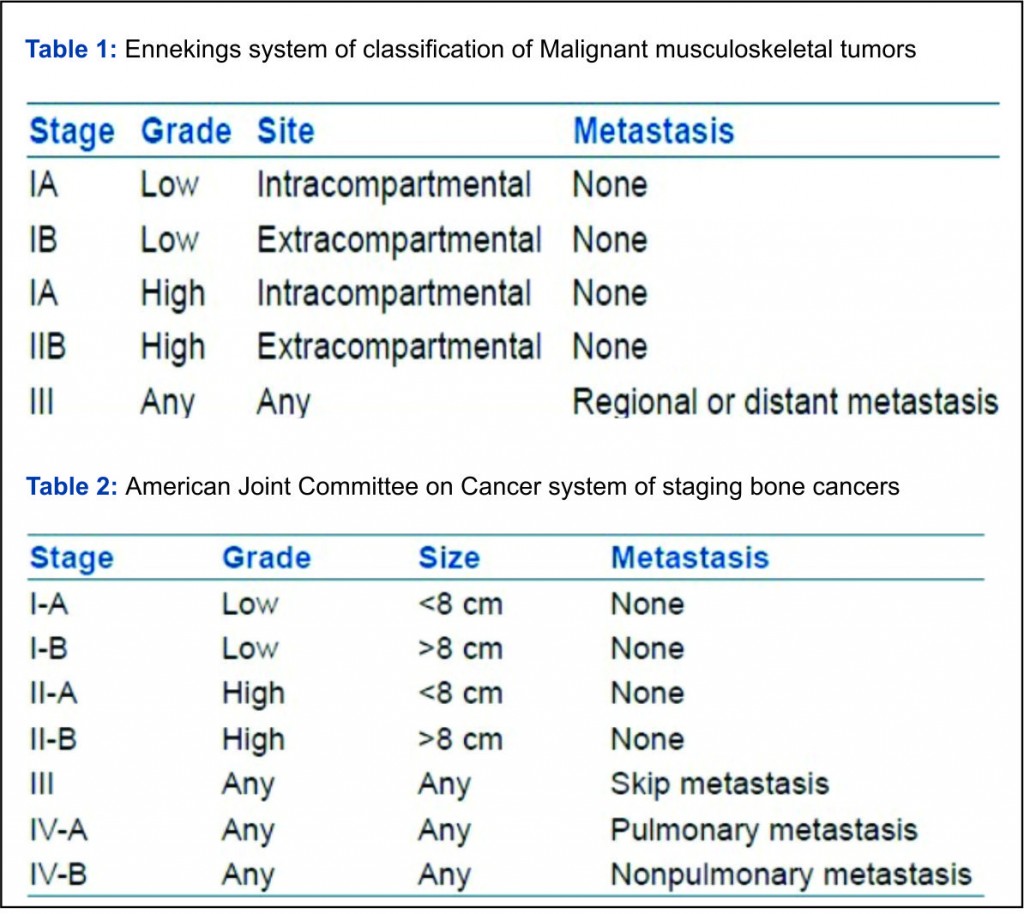
Cancer staging is a process to know the magnitude of the primary tumor and possible spread of the disease in a particular patient. It helps to understand the severity of the disease and hence the prognosis and thus aids in optimal treatment planning. Staging any cancer is therefore mandatory before starting its treatment. The most common site for metastasis in osteosarcoma is lung, followed by the skeletal system. At presentation, the reported incidence of lung metastasis is 15-20% whereas for skeletal metastasis it is 4%. Staging investigations includes High Resolution CT scan of thorax (plain) + Tc-99m methylene diphosphonate (Tc-99m MDP) Bone scan. Nowadays, 18 Fluoro Deoxy Glucose PET-CT scan is showing great promise as an alternative staging investigation. Plain chest radiograph can only detect large lung metastasis. For detection of early smaller lung lesions, a high resolution CT scan of thorax without contrast is recommended [21]. Typically metastases appear of soft tissue attenuation, dull, well circumscribed rounded lesions, more often in the periphery of the lung. Patients who present with metastatic pulmonary disease have a poorer prognosis. However, cure can be achieved in a small number of patients who respond well to chemotherapy and undergo pulmonary metastatectomy [22, 23]. (Tc-99m MDP) Triple-phase, whole-body bone scintigraphy still remains standard of care for determining the sites of metastatic disease in the skeletal system [24]. It may also detect skip lesions, although MRI is more accurate for this purpose. Whole-body turbo STIR MRI is also a reliable method for screening patients with suspected skeletal metastases. It is more specific than bone scan. This technique is also advantageous in that it reveals extraskeletal organ and soft tissue metastases [25]. Longer study time and cost are the limiting factors. Functional or metabolic imaging in form of 18 Fluoro Deoxy Glucose PET-CT scan is much more sensitive and specific than Tc-99m MDP bone scan in picking up the skeletal metastasis in osteosarcoma [26]. Moreover it gives valuable information on viable disease representation in proposed site for biopsy and some idea of the grade of the sarcoma. As it remains unaffected by presence of metallic prosthesis and radiation beam hardening artifacts, it is extremely valuable in detecting and defining a suspected recurrence [27]. However its scarce availability and prohibitive cost at present, makes it a difficult investigation to recommend in every case. Most popular staging system for bone and soft tissue sarcomas has been the Enneking’s staging system (Table 1). It is based on histological grade of sarcoma, local extent of disease i.e. intra or extra- compartmental involvement and presence or absence of metastasis [28]. American Joint committee on Cancer (AJCC) has also developed a staging system for sarcomas. (Table 2) It takes into the consideration the size of sarcoma, tumor grade, presence, and location of metastases [29].
Conclusion
Osteosarcoma is a high grade malignant disease which is fatal unless treated appropriately, in time. Effective treatment is available for this disease with a high cure rate. However, despite the availability of such treatment in developing countries, the cure rates for osteosarcoma are much lower as compared to the western population. One of the most significant points of failure is timely detection and diagnosis of this condition. Awareness of this disease and the knowledge of the vulnerable age group can go a long way in improving the prospects for osteosarcoma patients in developing countries. Time tested clinical skills along with readily available radiological imaging modalities and histopathology will help us reach accurate diagnosis and staging in most cases of osteosarcoma.
References
1. Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. 2006 Apr; 125(4):555-81.
2. Dorfman HA, Czerniak B. Bone Cancers. Cancer supplement. 1995;75(1):203–10.
3. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13.
4. Lisa Mirabello, Rebecca J. Troisi, and Sharon A. Savage. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Survei llance, Epidemiology, and En d R esults Program. Cancer. 2009 Apr 1; 115(7): 1531–1543.
5. Unni KK. Dahlin’s bone tumors: general aspects and data on 11,087 cases. 5. Philadelphia: Lippincott-Raven; 1996. pp. 143–83.
6. Huvos AG. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older tha n 60 years. Cancer . 1986 Apr 1;57(7):14 -42 9
7. Jhala DN, Eltoum I, Carroll AJ, et al. Osteosarcoma in a patient with McCune-Albright syndrome and Mazabraud’s syndro me: a case rep ort emp hasizin g the cytological and cy togenetic findings. Hum Pathol. 2003;34:1354-1357
8. Lisa Mirabello, Rebecca J. Troisi, and Sharon A. Savage. International osteosarcoma incidence patterns in children an d adolesce nts, middle ages, and elderly persons. Int J Cancer. 2009 Jul 1; 125(1): 229–234.
9. Lee RK1, Chu WC, Leung JH, Cheng FW, Li CK. Pathological fracture as the presenting feature in pediatric osteosarcoma . Pediatr Bloo d Cance r. 2013 Jul;60(7):1118-21.
10. Jeffree GM, Price CH, Sissons HA. The metastatic patterns of osteosarcoma. Br J Cancer. 1975 Jul; 32(1): 87–107
11. Yarmish G1, Klein MJ, Landa J, Lefkowitz RA, Hwang S. Imaging characteristics of primary osteosarcoma: nonconventional subtypes. Radiographics. 2010 Oct;30(6):1653-72.
12. Bacci G, Longhi A, Ferrari S, Briccoli A, Donati D, De Paolis M, Versari M. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori. 2004 Sep-Oct;90(5):4 78-8 4
13. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006 Mar 1;106(5): 1154-61.
14. Rubin DA. Magnetic resonance imaging: Practical considerations. In: Resnick D, Kransdorf MJ, editors. Bone and joint imaging. 3rd ed. Philadelphia Pennsylvania: Elsevier Saunders; 2005. pp. 118–32.
15. Bohndorf K, Reiser M, Lochner B, Feaux DL, Steinbrich W. Magnetic resonance imaging of primary tumors and tumor-like lesions of bone. Skeletal Radiol. 1986;15:511–7.
16. Skip Metastases in Osteosarcoma: Experience of the Cooperative Osteosarcoma Study Group JCO April 1, 2006 vol. 24 no. 10 1535-1541 Leo Kager, Andreas Zoubek, Ulrike Kastner et al.
17. Holscher HC, Bloem JL, Vanel D, Hermans J, Nooy MA, Taminiau AH, et al. Osteosarcoma: Chemotherapy induced changes at MR imaging. Radiology. 1992;182:839–44.
18. Uhl M, Saueressig U, van Buiren M, Kontny U, Niemeyer C, Köhler G, et al. Osteosarcoma: Preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion- and perfusion-weighted magnetic resonance imaging. Invest Radiol. 2006;41:618–23.
19. Liu PT, Valadez SD, Chivers FS, Roberts CC, Beauchamp CP. Anatomically Based Guidelines for Core Needle Biopsy of Bone Tumors: Implications for Limb-sparing Surgery Radiographics. 2007 Jan-Feb;27(1):189-205; discussion 206.
20. Jorda M1, Rey L, Hanly A, Ganjei-Azar P. Fine-needle aspiration cytology of bone: accuracy and pitfalls of cytodiagnosis. Cancer. 2000 Feb 25;90(1):47-54.
21. Picci P, Vanel D, Briccoli A et al. Computed tomography of pulmonary metastases from osteosarcoma: the less poor technique. A study of 51 patients with histological correlation. Ann Oncol 2001; 12: 1601–1604.
22. Rasalkar DD1, Chu WC, Lee V, Paunipagar BK, Cheng FW, Li CK. Pulmonary metastases in children with osteosarcoma: characteristics and impact on patient survival. Pediatr Radiol. 2011 Feb;41(2):227-36.
23. Bacci G, Picci P, Briccoli A, Avella M, Ferrari S, Femino FP, et al. Osteosarcoma of the extremity metastatic at presentation: results achieved in 26 patients treated with combined therapy (primary chemotherapy followed by simultaneous resection of the primary and met astatic lesions). Tumori. 1992;78:200–6.
24. Schneider R. Radionuclide technique. In: Resnick D, Kransdorf MJ, editors. Bone and joint imaging. 3rd ed. Philadelphia Pennsylvania: Elsevier Saunders; 2005. pp. 86–117.
25. Frat, Ali, Ağldere, Muhtesem , Gençoğlu, Arzu et al. Value of Whole-Body Turbo Short Tau Inversion Recovery Magnetic Resonance Imaging With Panoramic Table For Detecting Bone Metastases: Comparison With 99MTc-Methylene Diphosphonate Scintigraphy; Journal of Comp uter Assisted Tomography: January/February 2006 – Volume 30 – Issue 1 – pp 151-156
26. Byun BH, Kong CB, Lim SM et al. Comparison of (18) F-FDG PET/CT and (99 m)Tc-MDP bone scintigraphy for detection of bone metastasis in osteosarcoma. Skeletal Radiol. 2013 Dec; 42(12):1673-81.
27. Benz MR, Tchekmedyian N, Eilber FC et al. Utilization of positron emission tomography in the management of patients with sarcoma. Curr Opin Oncol 2009; 21: 345 –351.
28. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop. 1980;153:106–20.
29. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual (7th ed). New York, NY: Springer; 2010.
| How to Cite this article: Shah M, Anchan C. Evaluation of Osteogenic Sarcoma. Journal of Bone and Soft Tissue Tumors Jan-Apr 2016;2(1):8-12. |