Vol 5 | Issue 1 | Jan-April 2019 | page: 13-16 | Ismail Tawfeek Badr, Sanjiv Rampal Ahmed Shahin
Authors: Ismail Tawfeek Badr [1], Sanjiv Rampal [2], Ahmed Shahin [1]
[1] Department of Orthopaedic, Faculty of Medicine, Menoufia University, Faculty of Medicine, Egypt.
[2] Department of Orthopeaedic Department, Faculty of Medicine, Putra University, Faculty of Medicine, Malaysia.
Address of Correspondence
Dr. Ismail Tawfeek Badr,
Department of Orthopaedic, Faculty of Medicine, Menoufia University, Egypt.
E-mail: ismail.tawfeek@yahoo.com
Abstract
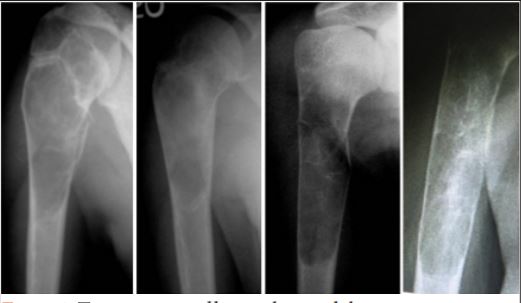
Introduction: Fibrous dysplasia (FD)is a benign pathological condition usually observed in the first three decades of life. A single bone may be involved either wholly or partially, or multiple bones may be affected, we aimed to use curettage and cementation as a control method of FDfibrous dysplasia of the proximal radius.
Methods: We describe our finding with the treatment of FDfibrous dysplasia of the proximal radius in five patients(four females and, one male), the mean age of 28.6 years (22 to –39 years). The lesions were in the metaphysis extending to the diaphysis. Persistent pain and pain after pathological fracture were the indications for surgical intervention. We used an extensile approach to expose the lesion then extended curettage using a high-speed burr and filling the cavity with bone cement. Functional outcome and radiological findings were monitored on follow-up visits.
Results: The mean follow-up period was 3.2 years (ranged from 2 years to 5 years).There waswereno recurrences and no patient sustained a fracture at the end of the filling cement. At the time of the last follow-up, all patients have excellent score(mean 27 points) according to the musculoskeletal tumor society scoring system.
Conclusion: Extended curettage and cementation provide a safe and reliable alternative for control of FDfibrous dysplasia of the proximal radius with little morbidity with little risk of recurrence and low incidence of complications.
Keywords: Fibrous dysplasia, Curettage, Cement.
References
1. Lichtenstein L. Fibrous dysplasia of bone. Arch Pathol 1942;33:777-816.
2. Chapurlat RD, Meunier PJ. Fibrous dysplasia of bone. Best Pract Res Clin Rheumatol 2000;14:385-98.
3. Boyce AM, Kelly MH, Brillante BA, Kushner H, Wientroub S, Riminucci M, et al. A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab 2014;99:4133-40.
4. Hart ES, Kelly MH, Brillante B, Chen CC, Ziran N, Lee JS, et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res 2007;22:1468-74.
5. Kelly MH, Brillante B, Collins MT. Pain in fibrous dysplasia of bone: Age-related changes and the anatomical distribution of skeletal lesions. Osteoporos Int 2008;19:57-63.
6. Smith SE, Kransdorf MJ. Primary musculoskeletal tumors of fibrous origin. Semin Musculoskelet Radiol 2000;4:73-88.
7. Kumar R, Madewell JE, Lindell MM, Swischuk LE. Fibrous lesions of bones. Radiographics 1990;10:237-56.
8. Guille JT, Kumar SJ, MacEwen GD. Fibrous dysplasia of the proximal part of the femur. Long-term results of curettage and bone-grafting and mechanical realignment. J Bone Joint Surg Am 1998;80:648-58.
9. Leet AI, Boyce AM, Ibrahim KA, Wientroub S, Kushner H, Collins MT, et al. Bone-grafting in polyostotic fibrous dysplasia. J Bone Joint Surg Am 2016;98:211-9.
10. Cabral CE, Guedes P, Fonseca T, Rezende JF, Cruz LC Jr., Smith J. Polyostotic fibrous dysplasia associated with intramuscular myxomas: Mazabraud’s syndrome. Skeletal Radiol 1998;27:278-82.
11. Leslie WD, Reinhold C, Rosenthall L, Tau C, Glorieux FH. Panostotic fibrous dysplasia. A new craniotubular dysplasia. Clin Nucl Med 1992;17:556-60.
12. Cutler CM, Lee JS, Butman JA, FitzGibbon EJ, Kelly MH, Brillante BA, et al. Long-term outcome of optic nerve encasement and optic nerve decompression in patients with fibrous dysplasia: Risk factors for blindness and safety of observation. Neurosurgery 2006;59:1011-7.
13. Leet AI, Magur E, Lee JS, Wientroub S, Robey PG, Collins MT, et al. Fibrous dysplasia in the spine: Prevalence of lesions and association with scoliosis. J Bone Joint Surg Am 2004;86-A:531-7.
14. Kumta SM, Leung PC, Griffith JF, Kew J, Chow LT. Vascularised bone grafting for fibrous dysplasia of the upper limb. J Bone Joint Surg Br 2000;82:409-12.
15. Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH, et al. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab 2003;88:4569-75.
16. Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and mcCune-albright syndrome. J Child Orthop 2007;1:3-17.
17. Adetayo OA, Salcedo SE, Borad V, Richards SS, Workman AD, Ray AO, et al. Fibrous dysplasia: An overview of disease process, indications for surgical management, and a case report. Eplasty 2015;15:e6.
18. Keijser LC, Van Tienen TG, Schreuder HW, Lemmens JA, Pruszczynski M, Veth RP, et al. Fibrous dysplasia of bone: Management and outcome of 20 cases. J Surg Oncol 2001;76:157-66.
19. Stanton RP, Ippolito E, Springfield D, Lindaman L, Wientroub S, Leet A. The surgical management of fibrous dysplasia of bone. Orphanet J Rare Dis 2012;7:S1.
20. Bryant DD 3rd, Grant RE, Tang D. Fibular strut grafting for fibrous dysplasia of the femoral neck. J Natl Med Assoc 1992;84:893-7.
21. Shih HN, Chen YJ, Huang TJ, Hsu KY, Hsu RW. Treatment of fibrous dysplasia involving the proximal femur. Orthopedics 1998;21:1263-6.
22. Weiland AJ, Moore JR, Daniel RK. Vascularized bone autografts. Experience with 41 cases. Clin Orthop Relat Res 1983;174:87-95.
23. Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, et al. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat Rev 2010;36:1-7.
24. Gitelis S, Mallin BA, Piasecki P, Turner F. Intralesional excision compared with en bloc resection for giant-cell tumors of bone. J Bone Joint Surg Am 1993;75:1648-55.
25. Keçeci B, Küçük L, Isayev A, Sabah D. Effect of adjuvant therapies on recurrence in aneurysmal bone cysts. Acta Orthop Traumatol Turc 2014;48:500-6.
26. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;286:241-6.
27. Stephenson RB, London MD, Hankin FM, Kaufer H. Fibrous dysplasia. An analysis of options for treatment. J Bone Joint Surg Am 1987;69:400-9.
28. Weiland AJ, Phillips TW, Randolph MA. Bone grafts: A radiologic, histologic, and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast Reconstr Surg 1984;74:368-79.
29. Rosario MS, Hayashi K, Yamamoto N, Takeuchi A, Miwa S, Taniguchi Y, et al. Functional and radiological outcomes of a minimally invasive surgical approach to monostotic fibrous dysplasia. World J Surg Oncol 2017;15:1..
| How to Cite this article: Badr I T, Rampal S, Shahin A. Preliminary Results of Curettage and Cementation in the Treatment of Fibrous Dysplasia of the Proximal Radius. Journal of Bone and Soft Tissue Tumors Jan-Apr 2019;5(1): 13-16. |

Like this:
Like Loading...